A product for the treatment of hemophilia b
A technology with a product and purpose, applied in the field of products for the treatment of hemophilia B, can solve the problems of inability to achieve knock-in, limit the loading capacity, etc., and achieve the effects of increasing the insertable capacity, efficient integration, and reducing immune responses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0065] The present invention will be further described in detail with reference to the following specific embodiments and accompanying drawings, and the protection content of the present invention is not limited to the following embodiments. Variations and advantages that can occur to those skilled in the art without departing from the spirit and scope of the inventive concept are included in the present invention, and the appended claims are the scope of protection. The process, conditions, reagents, experimental methods, etc. for implementing the present invention, except for the contents specifically mentioned below, are all common knowledge and common knowledge in the field, and the present invention has no special limited contents. Conditions were as described in Sambrook et al., Molecular Cloning, Laboratory Manual (New York: Cold Spring Harbor Laboratory Press, 1989), or as suggested by the manufacturer.
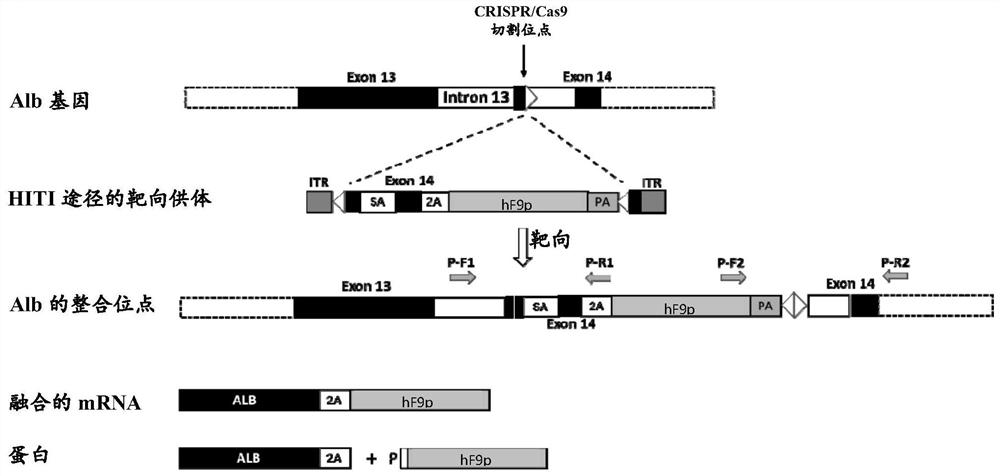
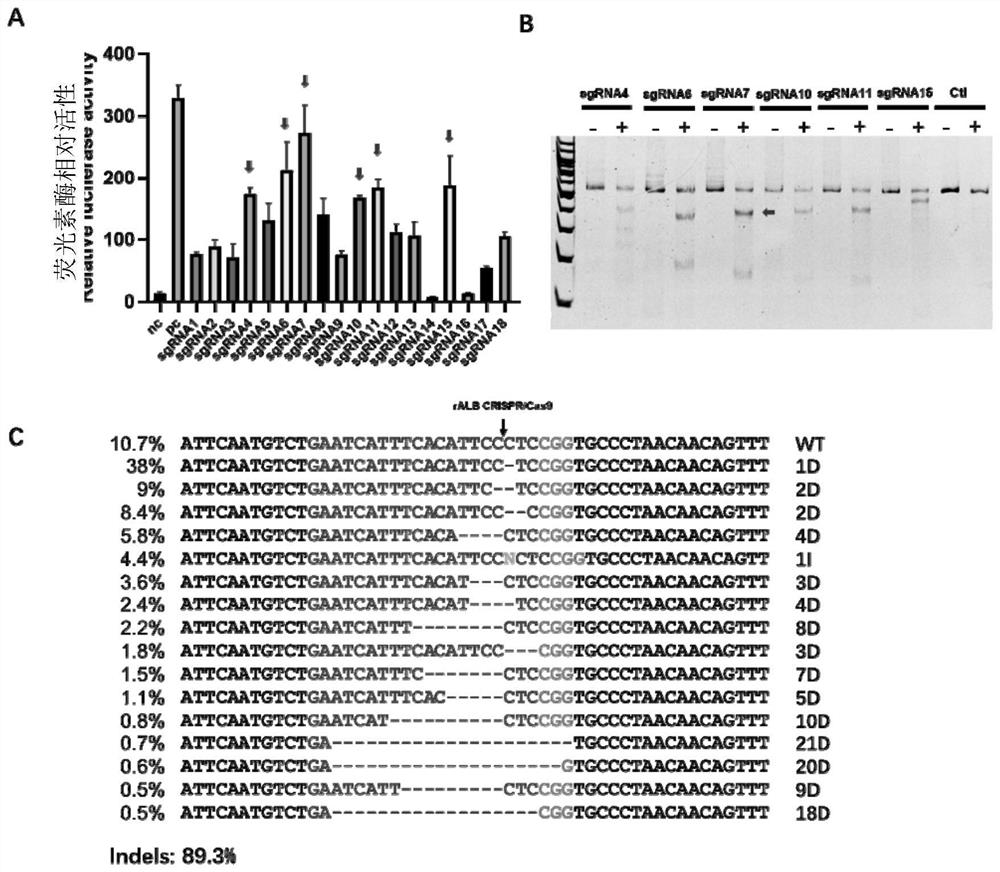
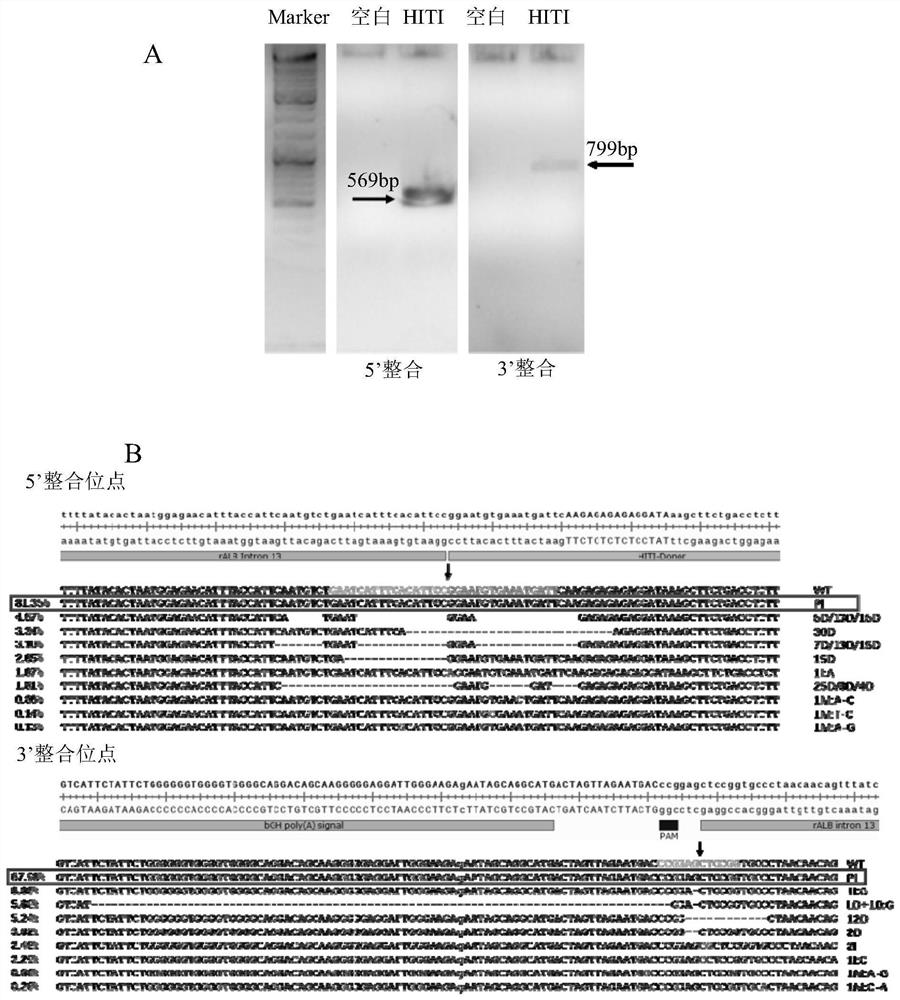
[0066] like figure 1 As shown, the present invention is based o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com