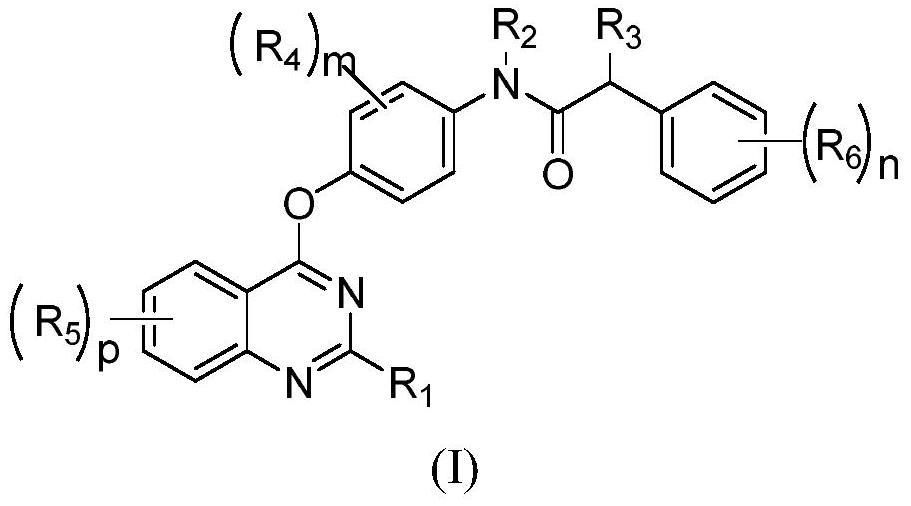
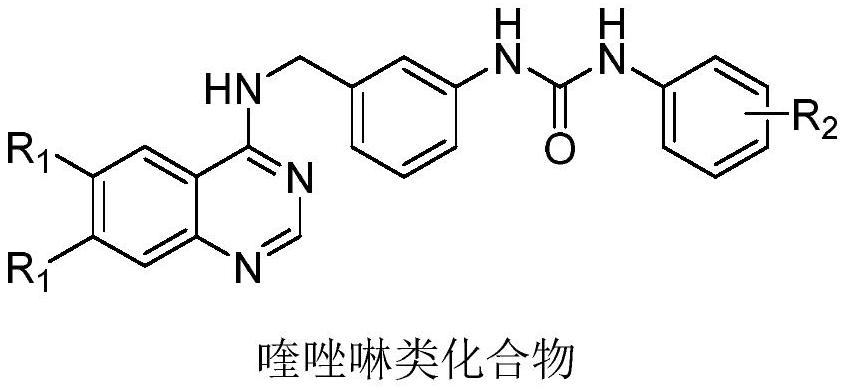
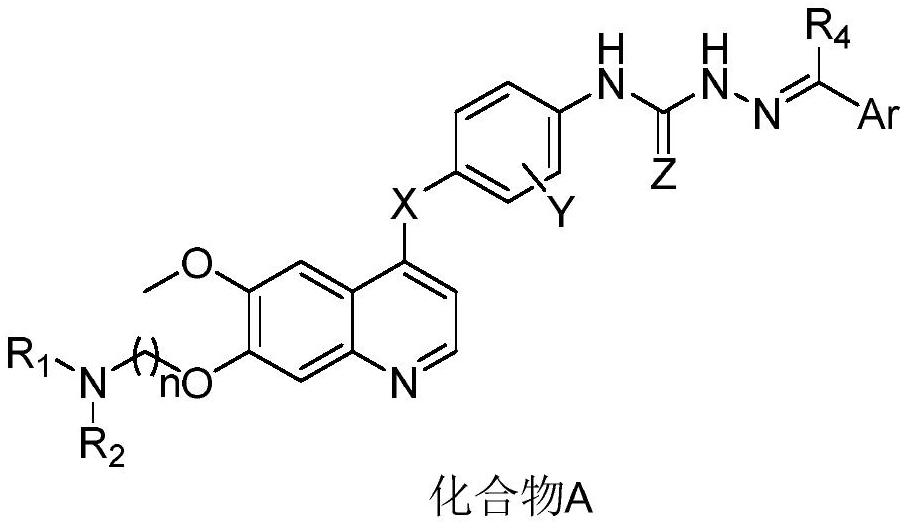
Quinazoline compound and application thereof
A technology of quinazolines and compounds, which is applied in the field of medicine and can solve problems such as structural differences of compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Embodiment 1: the preparation of compound 1
[0106]
[0107] Step 1: Add intermediate a (20g, 101mmol), ammonium formate (80mL) and formic acid (8mL) into a 250mL one-necked bottle, heat to 135°C and stir for 5 hours. Pour the reaction solution into a water bath to precipitate the product, and filter to obtain 23 g of the product.
[0108] Step 2: Add intermediate b (5g, 24.3mmol) to SOCl 2 (50mL), heated to reflux for 3h. After the reaction was completed, 5.3 g of the product was obtained by precipitation.
[0109] Step 3: Dissolve intermediate c (5.3g, 23.7mmol) and intermediate d (2.8g, 25.69mmol) in butanone (100mL), add NaOH (3g, 75mmol), H 2 O (20mL, 1.1mol) and TBAB (4g, 12mmol) were heated to 100°C for 4h. After the reaction, the product was purified by column chromatography to obtain 2.6g of the product.
[0110] Step 4: Dissolve intermediate e (100mg, 0.337mmol) and intermediate f (50mg, 0.325mmol) in THF (10mL), add DIPEA (200mg, 1.55mmol) and HBTU (1...
Embodiment 2
[0112] Embodiment 2: the preparation of compound 2
[0113]
[0114] The preparation method of intermediate e is the same as in Example 1. The preparation method of compound 2 is as follows:
[0115] Step 1: Dissolve intermediate e (100mg, 0.337mmol) and intermediate f (54mg, 0.325mmol) in THF (10mL), add DIPEA (200mg, 1.55mmol) and HBTU (130mg, 0.343mmol) and stir at room temperature for 6 100 mg of compound 2 was obtained by column chromatography.
[0116] 1H NMR(600MHz,DMSO-d6)δ10.23(s,1H),8.53(s,1H),7.71–7.66(m,2H),7.55(s,1H),7.37(s,1H),7.30– 7.26(m,2H),7.25–7.22(m,2H),6.92–6.88(m,2H),3.99(s,3H),3.97(s,3H),3.74(s,3H),3.59(s, 2H). MS: 445.16.
Embodiment 3
[0117] Embodiment 3: the preparation of compound 3
[0118]
[0119] The preparation method of intermediate e is the same as in Example 1. The preparation method of compound 3 is as follows:
[0120] Step 1: Dissolve intermediate e (100mg, 0.337mmol) and intermediate f (54mg, 0.325mmol) in THF (10mL), add DIPEA (200mg, 1.55mmol) and HBTU (130mg, 0.343mmol) and stir at room temperature for 6 Hours, column chromatography obtained 100 mg of compound 3 product.
[0121] 1H NMR (600MHz, DMSO-d6) δ10.17(s,1H),8.53(s,1H),7.74–7.66(m,2H),7.55(s,1H),7.38(s,1H),7.28– 7.22(m,4H),6.99(dd,J=8.1,1.1Hz,1H),6.92(td,J=7.4,1.1Hz,1H),3.99(s,3H),3.97(s,3H),3.79 (s,3H),3.66(s,2H).MS:445.16.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com