Anti-CD123 antibody and application thereof
An antibody and antigen technology, applied in the field of immunology, can solve the problems of poor specificity and low affinity of CD123 antibody, and achieve the effect of strong binding specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0123] Example 1: Screening of Mouse Hybridoma Monoclonal Antibodies
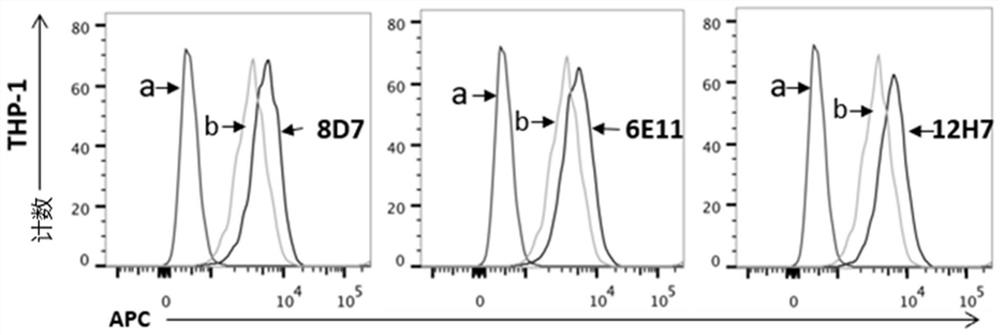
[0124] Using human CD123 protein as the immunogen, Balb / c mice were immunized by intraperitoneal injection, booster immunization was carried out at the 3rd week and the 5th week after the initial immunization, and the mouse tail blood was collected on the 8th day after the booster immunization , stand at room temperature for 1 hour, centrifuge at 12,000 rpm for 10 minutes at 4°C, collect serum, and dilute with PBS to different concentrations: 1:200, 1:400, 1:800, 1:1600, 1:3200, 1:6400, 1 : 12800. Collect THP-1 cells, wash once with PBS, count the cells, use 1×10 for each sample 6 Add 100 μl of serum with different dilutions to the cells, and in the negative control group, replace the antiserum with the serum of unimmunized mice, incubate at 4°C for 1 hour, wash twice with PBS; add 2 μl of PE-labeled rat anti-mouse IgG antibody , and incubate at 4°C in the dark for 40 minutes; cells were resuspended in 50...
Embodiment 2
[0126] Embodiment 2: Ascites preparation and purification
[0127] Wash hybridoma cells with sterile PBS solution, 5x10 6 The cell volume of 0.5ml / mouse was intraperitoneally injected into the liquid paraffin presensitized Balb / c mice. Collect ascites after 7 to 10 days, room temperature 3000rpm, 10min, collect supernatant. The antibody was crudely purified with saturated ammonium sulfate with a final concentration of 33%. The method was to take 1 part of ascitic fluid, add 1 part of PBS, add 1 part of saturated ammonium sulfate dropwise, stir while adding, overnight at 4°C, and centrifuge at 10,000 rpm for 10 minutes to remove the supernatant , Dissolve the precipitate with a small amount of PBS, dialyze with PBS for 24 hours at 4°C to desalt, and change the medium 3 times during this period. According to the purification manual provided by GE, the purified antibody was further purified by using the AKTA protein purification system and 1ml Protein G purification prepacked c...
Embodiment 3
[0128] Example 3: Monoclonal antibody titer detection
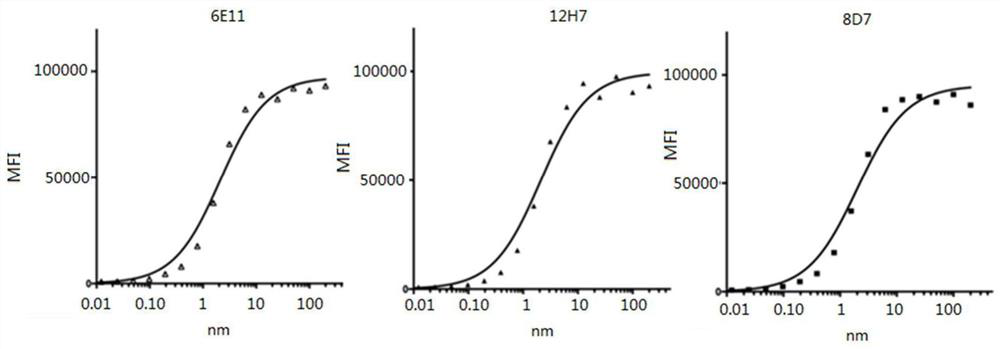
[0129] Fluorescently label the pure antibody, PE directly labeled with 6E11, 8D7, 12H7 antibody. Labeled antibodies at final concentrations of 400nM, 200nM, 100nM, 50nM, 25nM, 12.5nM, 6.25nM, 3.2nM, 1.6nM, 0.8nM, 0.4nM, 0.2nM, 0.1nM, 0.05nM, 0.025nM, 0.0125nM, 0.0061nM, 0.003nM and 2.5×10 5 3T3 (transfected with CD123) were incubated at room temperature for 30 min, and protected from light. Centrifuge at 1800 rpm for 10 min, discard the supernatant, wash the cells with PBS, repeat three times, resuspend the cells in 400 μl of PBS, measure the fluorescence intensity by FACS, and calculate the Mean value. The Kd value of the antibody was calculated with the data analysis software GraphPad Prism 5. The result is as figure 1 As shown, the Kd values of 12H7, 6E11, and 8D7 are 2.09×10 -9 M, 2.10×10 -9 M, 2.01×10 -9 M.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com