Compounds useful in treatment of autoimmune and inflammatory disorders
A compound and hydrate technology, applied in allergic diseases, anti-inflammatory agents, drug combinations, etc., can solve problems such as adverse side effects, discomfort, toxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0339] Synthetic methods for incorporating radioisotopes into organic compounds are applicable to the compounds of the invention and are well known in the art. Some synthetic methods for incorporating tritium activity levels into target molecules include the following:
[0340] A. Catalytic reduction with tritium gas: This method usually produces high specific activity products and requires halogenated or unsaturated precursors.
[0341] B. Using sodium borohydride [ 3H] Reduction: This method is rather cheap and requires precursors containing reducible functional groups such as aldehydes, ketones, lactones, esters, etc.
[0342] C. With lithium aluminum hydride [ 3 H] Reduction: This method provides products with almost theoretical specific activities. It also requires precursors containing reducible functional groups, such as aldehydes, ketones, lactones, esters, etc.
[0343] D. Tritium Gas Exposure Labeling: This method involves exposing precursors containing exchangea...
example 1
[0349] Example 1: Synthesis of compounds of the present invention
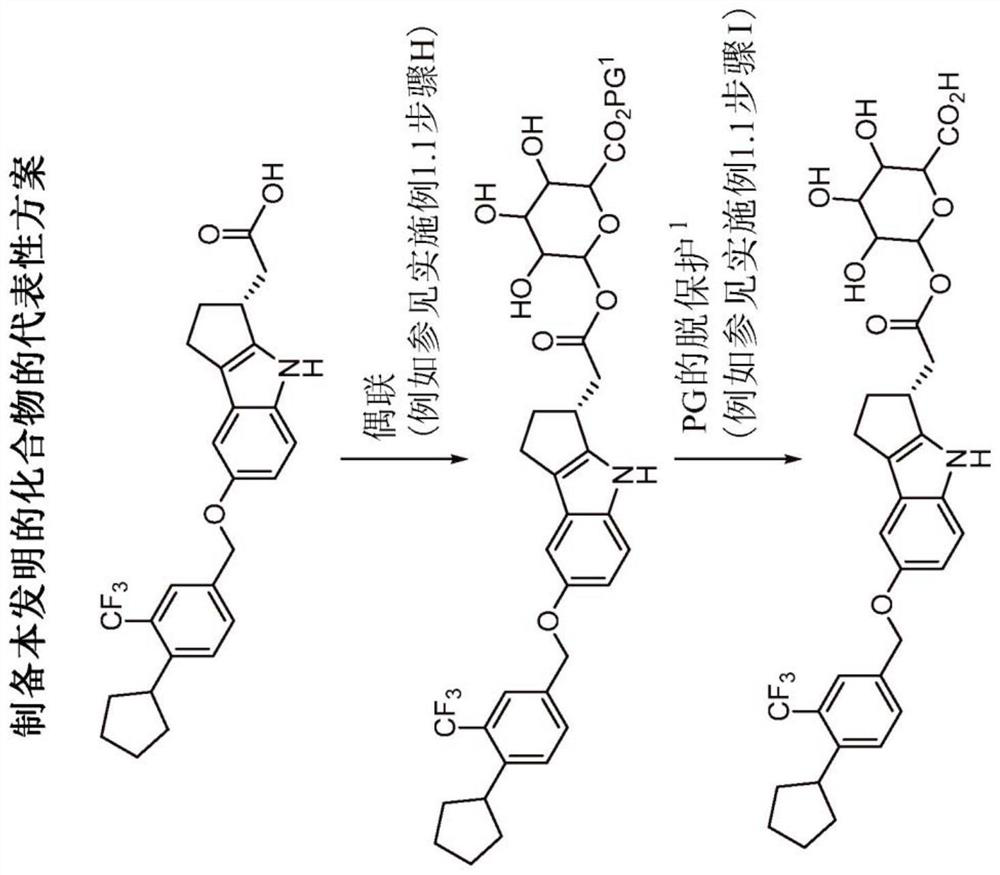
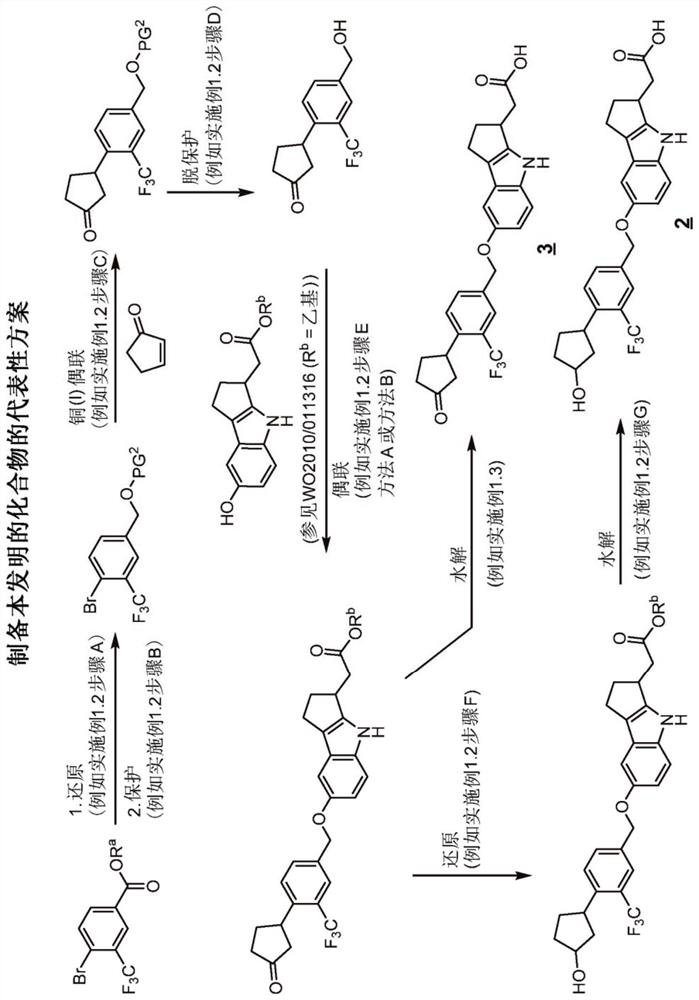
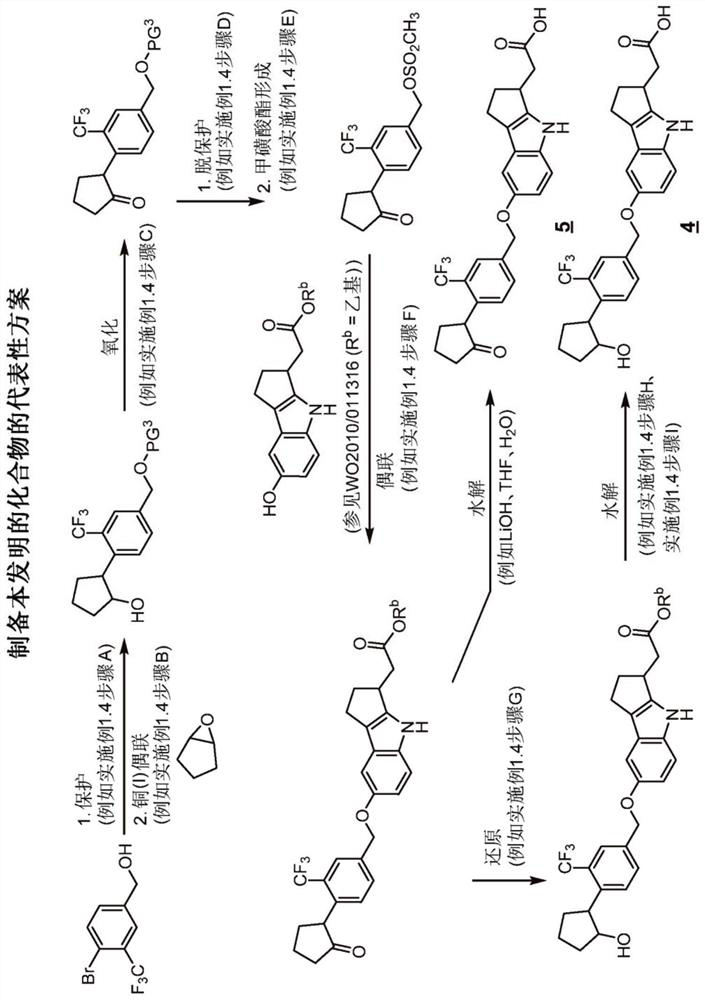
[0350] exist Figure 1A , 1B Illustrative syntheses of compounds of the invention are shown in and 1C.
[0351] The compounds of the present invention and their synthesis are further illustrated by the following examples. The following examples are provided to further define the invention without restricting the invention to the details of these examples. according to Professional (version 17.0.0.206) names the compounds described herein (above and below). In certain instances, generic names are used, and it is understood that those skilled in the art will recognize such generic names.
[0352] Chemistry: Proton NMR was recorded on a Bruker Avance-400 equipped with QNP (Quadruple Nucleus Probe) or BBI (Broad Band Inverse) and z-gradient ( 1 H NMR) spectrum. Proton NMR was also recorded on a Bruker Avance-500 equipped with BBI (Broadband Inverse) and z-gradient ( 1 H NMR) spectrum. Chemical shifts are ...
Embodiment 11
[0354] Example 1.1: (2S, 3S, 4S, 5R)-6-(2-((R)-7-((4-cyclopentyl-3-(trifluoromethyl)benzyl)oxy)-1 , 2,3,4-tetrahydrocyclopenta[b]indol-3-yl)acetoxy)-3,4,5-trihydroxytetrahydro-2H-pyran-2-carboxylic acid (compound 1) preparation.
[0355] Step A: Preparation of 1-cyclopentyl-2-(trifluoromethyl)benzene.
[0356] To a 50 L three necked round bottom flask equipped with mechanical stirrer, thermocouple and nitrogen inlet was added anhydrous THF (35 L) and cooled to 0-5 °C. Iron (III) chloride (2.7 kg, 0.15 equiv) was added to the flask in portions over 30-60 minutes and stirred for 15-30 minutes, resulting in a clear green solution. Under a nitrogen atmosphere, THF (87.5 L) and magnesium turnings (4.05 kg, 1.5 equiv) were added to a dry 100 gallon glass-lined reactor and cooled to 0-5°C. FeCl 3 The solution in THF was added to the mixture of THF and magnesium at a rate that kept the internal temperature below 10°C. To the resulting yellow / green mixture was added TMEDA (15.5 kg...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com