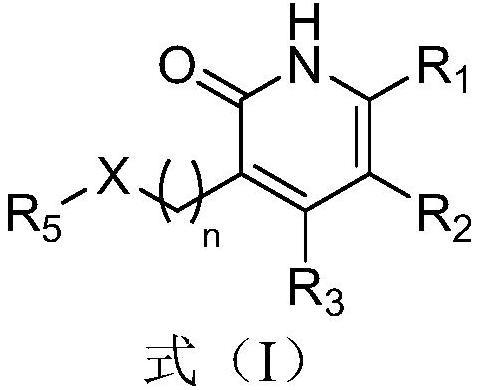
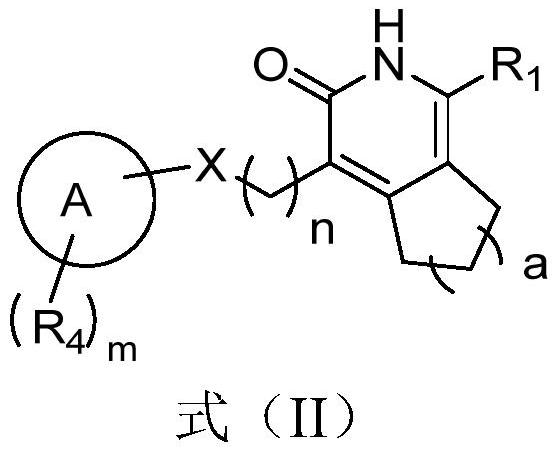
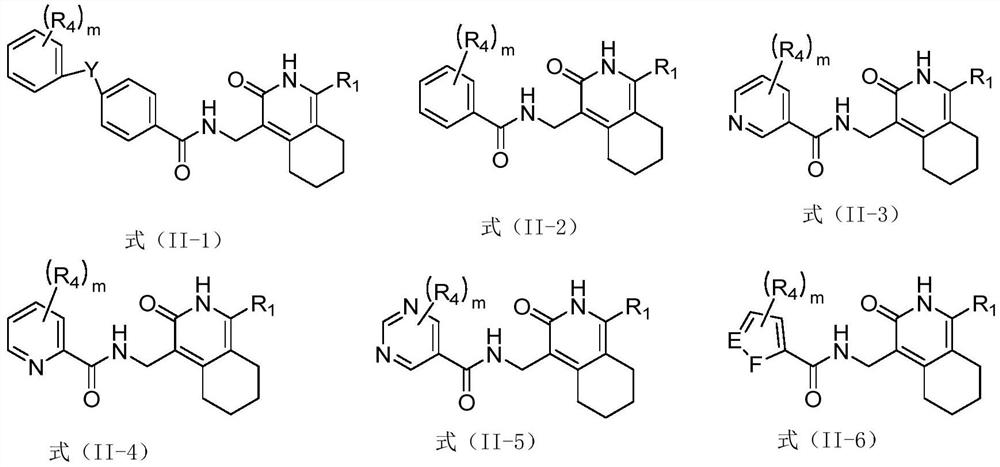
Pyridone derivative and application thereof in preparation of medicine for preventing and/or treating tuberculosis caused by mycobacterium tuberculosis
A pharmacy and compound technology, applied in the direction of antibacterial drugs, active ingredients of heterocyclic compounds, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1 Preparation of 4'-fluoro-N-((1-methyl-3-oxo-2,3,5,6,7,8-hexahydroisoquinolin-4-yl)methyl)-[ 1,1'-biphenyl]-4-carboxamide (Compound 1)
[0086] Compound 1 was synthesized according to the following route:
[0087]
[0088] 1.1. Synthesis of acetylcyclohexanone (i.e. intermediate 1m):
[0089] Mix cyclohexanone (29.50g) and morpholine (31.45g) and add solvent toluene (150mL), add p-toluenesulfonic acid (0.53g) while stirring, and slowly heat to 120°C. Use a water separator to separate the water produced by the reaction. When 6.00 mL of water is separated, stop the reaction, and gradually cool the reaction system to room temperature, and remove the solvent under reduced pressure to obtain a dark yellow viscous liquid. Dissolve the liquid in dichloromethane, stir and cool down to 0 °C, add 50.00 mL of triethylamine, and stir for 5 min. Dilute 24.00 mL of acetyl chloride with dichloromethane and slowly drop into the reaction system, a large amount of white sm...
Embodiment 2
[0097] Example 2 Preparation of N-((1-methyl-3-oxo-2,3,5,6,7,8-hexahydroisoquinolin-4-yl)methyl)-4'-(trifluoro Methoxy)-[1,1'-biphenyl]-4-carboxamide (Compound 2)
[0098]
[0099] Synthesize intermediate 1a according to the method of Example 1, and then refer to the method of step 1.4 of Example 1, replacing the raw material with 4'-(trifluoromethoxy)-[1,1'-biphenyl]-4-carboxylic acid , Compound 2 was obtained.
[0100] 1 H NMR (400MHz, DMSO-d 6 )δ11.51(s, 1H), 8.49(t, J=4.7Hz, 1H), 8.11(d, J=1.8Hz, 2H), 7.91-7.75(m, 4H), 7.47(d, J=8.3 Hz,2H),4.36(d,J=4.6Hz,2H),2.70(s,2H),2.38(s,2H),2.11(s,3H),1.63(s,4H).ESI-MS:m / z[M+Na] + calculated for 479.2, found 479.1.
Embodiment 3
[0101] Example 3 Preparation of 4'-(dimethylamino)-N-((1-methyl-3-oxo-2,3,5,6,7,8-hexahydroisoquinolin-4-yl)methanol base)-[1,1'-biphenyl]-4-carboxamide (Compound 3)
[0102]
[0103] Synthesize intermediate 1a according to the method of Example 1, and then refer to the method of step 1.4 of Example 1, replacing the raw material with 4'-(dimethylamino)-[1,1'-biphenyl]-4-carboxylic acid to prepare Compound 3 was obtained.
[0104] 1 H NMR (400MHz, DMSO-d 6 )δ11.51(s, 1H), 8.37(t, J=4.8Hz, 1H), 7.90(d, J=8.3Hz, 2H), 7.69(d, J=8.1Hz, 2H), 7.27(t, J=8.1Hz, 1H), 7.00-6.91(m, 2H), 6.76(dd, J=8.4, 2.4Hz, 1H), 4.36(d, J=4.7Hz, 2H), 2.96(s, 6H), 2.72(s,2H),2.38(s,2H),2.12(s,3H),1.63(s,4H).ESI-MS:m / z[M+Na] + calculated for 438.2, found 438.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com