Application of meisoindigo in preparation of medicine for treating acute lung injury
A technology for acute lung injury and methylisoindigo, which can be used in drug combinations, pharmaceutical formulations, organic active ingredients, etc., to solve problems such as reducing mortality and unprincipled treatment of COVID19
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1 Pharmacological test of meisoindigo protecting acute lung injury
[0024] 1) Establishment of acute lung injury model in mice
[0025] SPF grade ICR mice, male, 8-10 weeks old, weighing 25±5g. Housed at 22°C, 12 hour light-dark cycle (lights on at 8 am), with free access to food and water. The mice were tested after 7 days of adaptation to the environment. The age and body weight of each group of experimental animals were the same. After the last administration, a single intraperitoneal injection of LPS (10 mg / kg) was used to reproduce the endotoxin-induced ALI model. After 12 hours of modeling, the mice were sacrificed, and lung tissue and blood samples were taken.
[0026] 2) Dosage and method of administration
[0027] The doses of meisoindigo were low dose group: 2.5mg / kg; middle dose group: 5mg / kg; high dose group: 10mg / kg. Three days before modeling, the drug was administered by intragastric administration every day.
[0028] 3) Blood flow measure...
Embodiment 2
[0055] Example 2 Meisoindigo protects the results and analysis of acute lung injury
[0056] 1) Effects of meisoindigo on lung injury and pulmonary blood flow in ALI mice
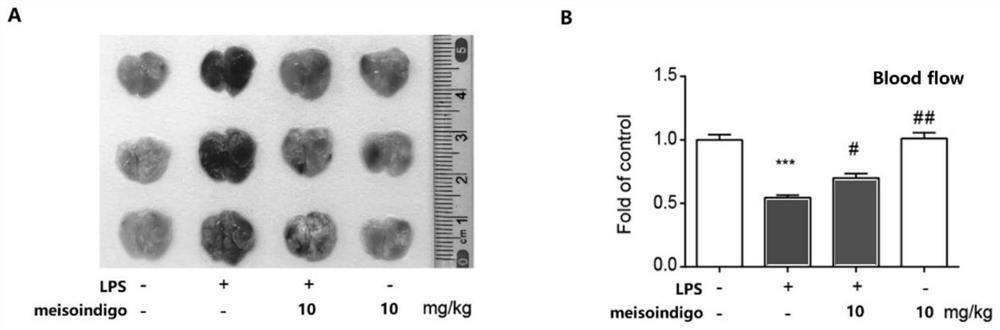
[0057] like figure 1 As shown in .A, the lungs of the mice in the normal group were smooth, uniformly pink, and soft. Injection of LPS caused hemorrhage, swelling and partial necrosis in mice. Meisoindigo significantly improved lung tissue stasis and damage. Moreover, administration of 10 mg / kg meisoindigo to mice alone did not show toxicity to the lung tissue of mice.
[0058] Decreased blood flow to lung tissue is one of the typical features of lung injury. figure 1 .B shows that the treatment of meisoindigo can significantly improve the decrease of blood flow in lung tissue caused by blood stasis, and the results have significant differences. At the same time, administration of 10 mg / kg meisoindigo to mice alone had no effect on blood flow in lung tissue.
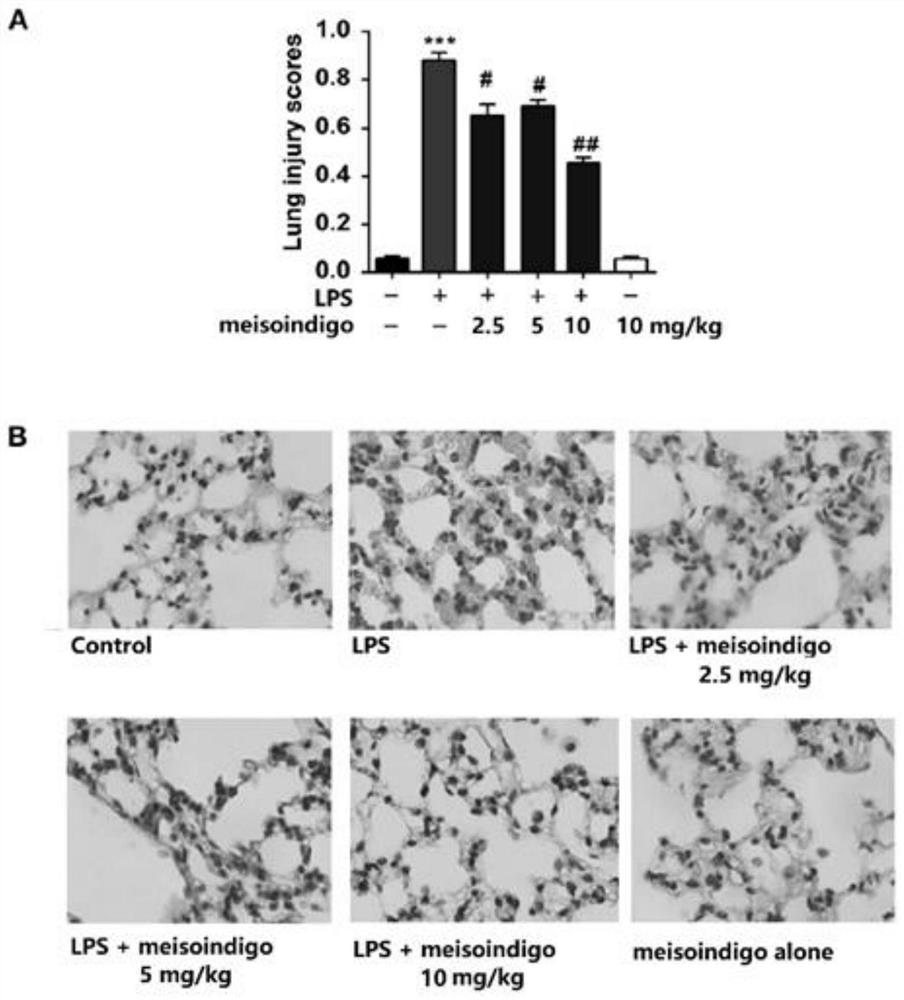
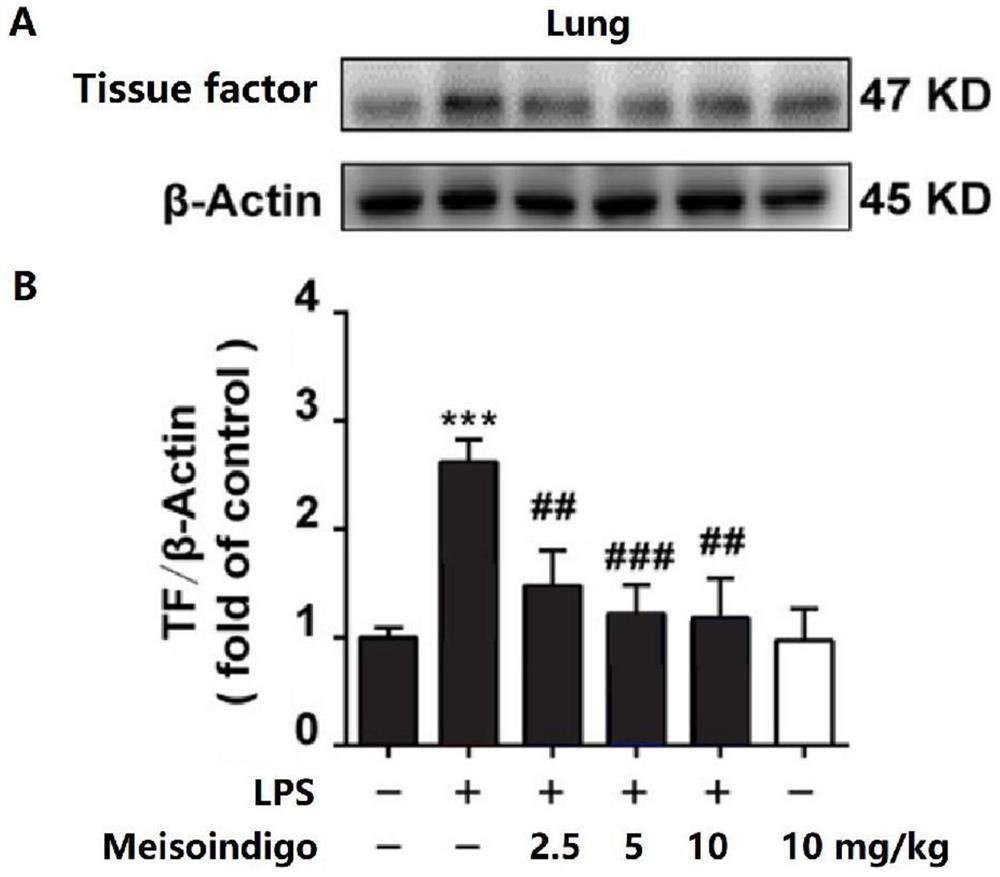
[0059] 2) Effect of meisoindigo on the hi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com