Application of naphthalimide-polyamine derivatives combined with cyclosporin a in the preparation of antitumor drugs
An anti-tumor drug, naphthalimide technology, applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problems of obvious toxic and side effects, achieve no toxic and side effects, improve anti-tumor effect, and improve anti-tumor effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
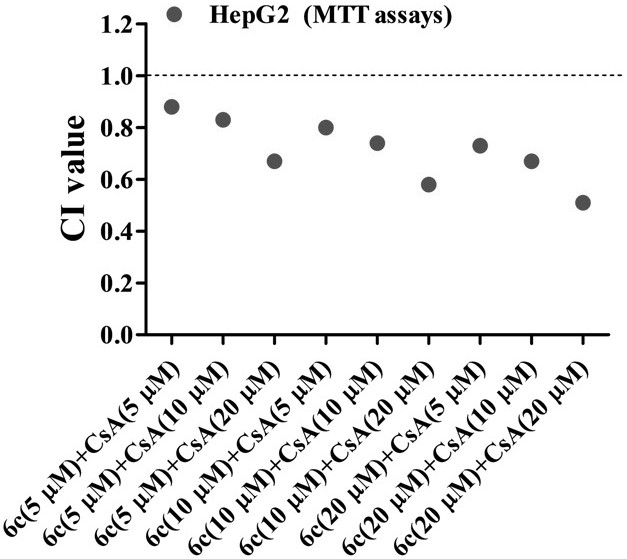
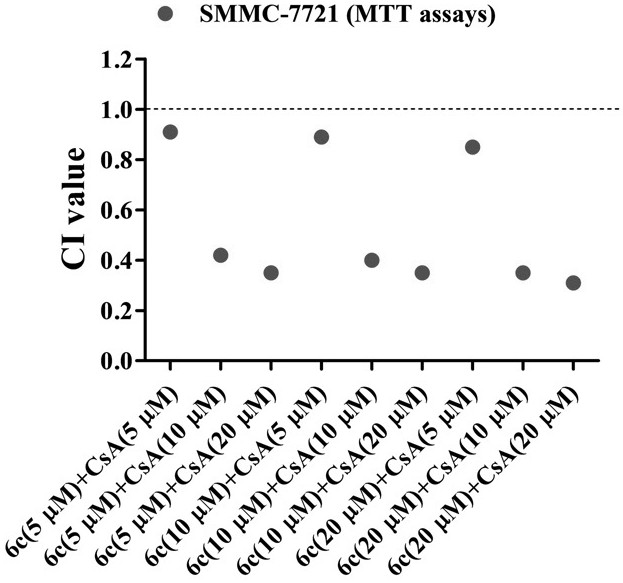
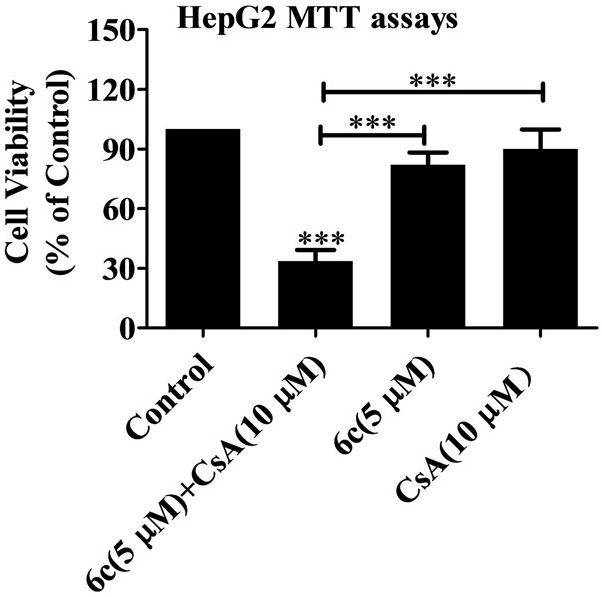
[0062] Example 1: In vitro biological activity evaluation of naphthalimide-polyamine derivative 6c and cyclosporine A (CsA) combined use
[0063] Test one:
[0064] Human liver cancer cells HepG2 and SMMC-7721 in the logarithmic growth phase were respectively taken, and the cancer cells were digested with trypsin, centrifuged at a speed of 1000r / min for 5 minutes, the supernatant was discarded, suspended in the complete medium, and the number of cells was counted by a hemocytometer. Density is 8×10 3 The inoculum amount of cells / well was inoculated in a 96-well plate, and after 24 hours of adherent growth, naphthalimide-polyamine derivative 6c, cyclosporine A (CsA) was added alone or in combination. The final concentration of single drug is CsA (5 μM), CsA (10 μM), CsA (20 μM), 6c (5 μM), 6c (10 μM), 6c (20 μM); the final concentration of combined drug is 6c (5 μM) + CsA (5 μM), 6c(5μM)+CsA(10μM), 6c(5μM)+CsA(20μM), 6c(10μM)+CsA(5μM), 6c(10μM)+CsA(10μM), 6c(10μM)+CsA(20μM), ...
Embodiment 2
[0089] Example 2: In vivo biological activity evaluation of naphthalimide-polyamine derivative 6c and cyclosporine A (CsA) combined use
[0090] Test nine:
[0091] H22 liver cancer cells from mice (Balb / c mice) were extracted from the abdominal cavity, centrifuged at 1000r / min for 5 minutes, the supernatant was discarded, and washed with pre-cooled saline until no blood remained, usually three times. Each mouse (the mouse is 6 weeks old and weighs about 20g) was inoculated with H22 cells in the tail vein, and the number of cells was 2×10 6. Tumor-bearing mice were divided into four groups, namely blank control group, naphthalimide-polyamine derivative 6c (2mg / kg / day) group, cyclosporine A (CsA, 25mg / kg / day) group and combined Medication group (6c2mg / kg / day+CsA 25mg / kg / day). Naphthalimide-polyamine derivative 6c was administered by tail vein injection, and cyclosporin A (CsA) was administered by intragastric administration for 12 consecutive days. After the experiment, acc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com