Method for synthesizing five-membered oxygen-containing heterocyclic compound
A technology of heterocyclic compounds, synthesis methods, applied in the field of organic catalysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
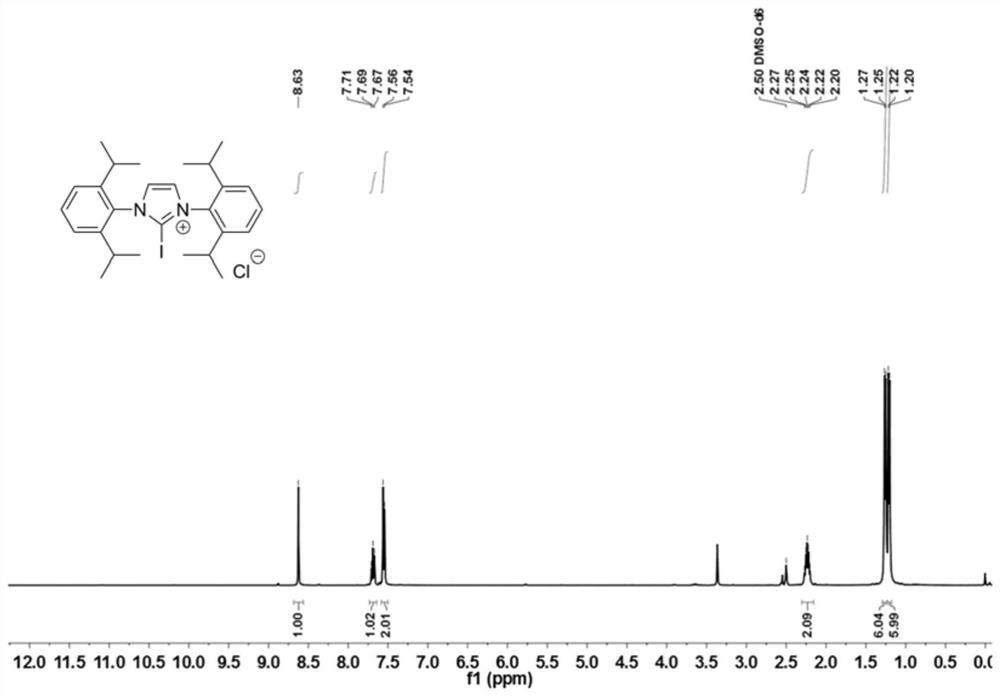
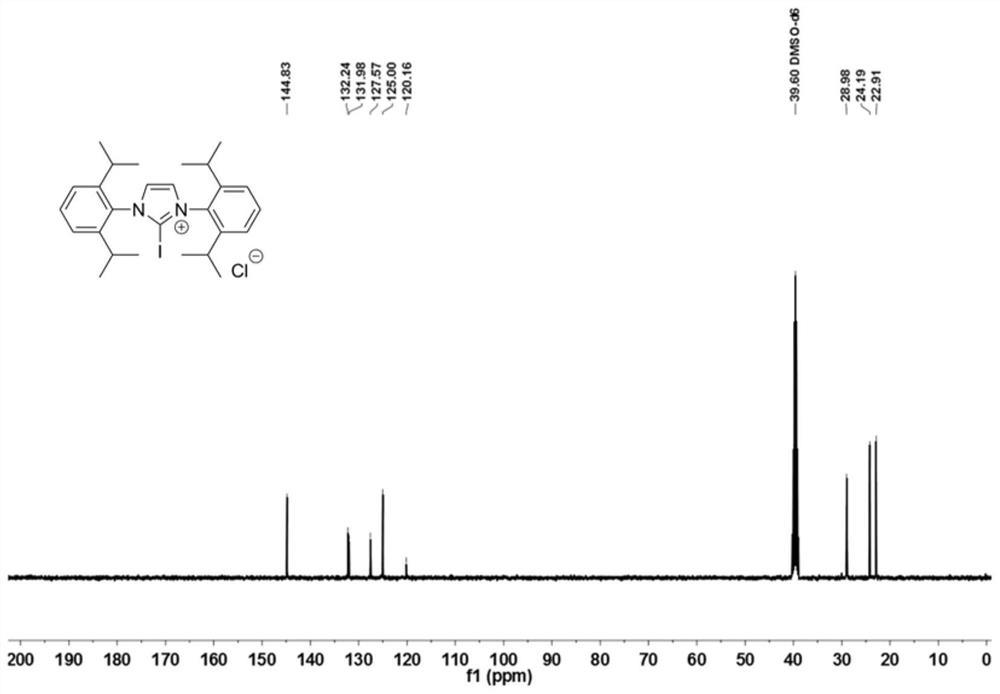
[0077] The 250mL reaction bottle was fully dried and filled with inert gas protection. Add 1,3-(2,6-diisopropylphenyl)imidazolium chloride (4.25g, 10mmol, 1.0equiv.), and add 100mL of anhydrous dichloromethane to dissolve it, then add N-iodo Succinimide (2.36g, 10.5mmol, 1.05equiv.), after stirring at 40°C for 2 hours, the reaction solution was washed with deionized water 50×3, the organic phases were combined, dried, and the solvent was removed under reduced pressure to obtain catalyst 1 , as light yellow powder, yield 98%. 1 HNMR (400MHz, DMSO-d 6 )δ8.63(s, 2H), 7.69(t, J=7.8Hz, 2H), 7.55(d, J=7.8Hz, 4H), 2.24(p, J=6.7Hz, 6H), 1.23(dd, J=19.3,6.8Hz,12H). 13 C NMR (101MHz, DMSO-d 6 )δ144.83, 132.24, 131.98, 127.57, 125.00, 120.16, 39.60, 28.98, 24.19, 22.91.
Embodiment 2
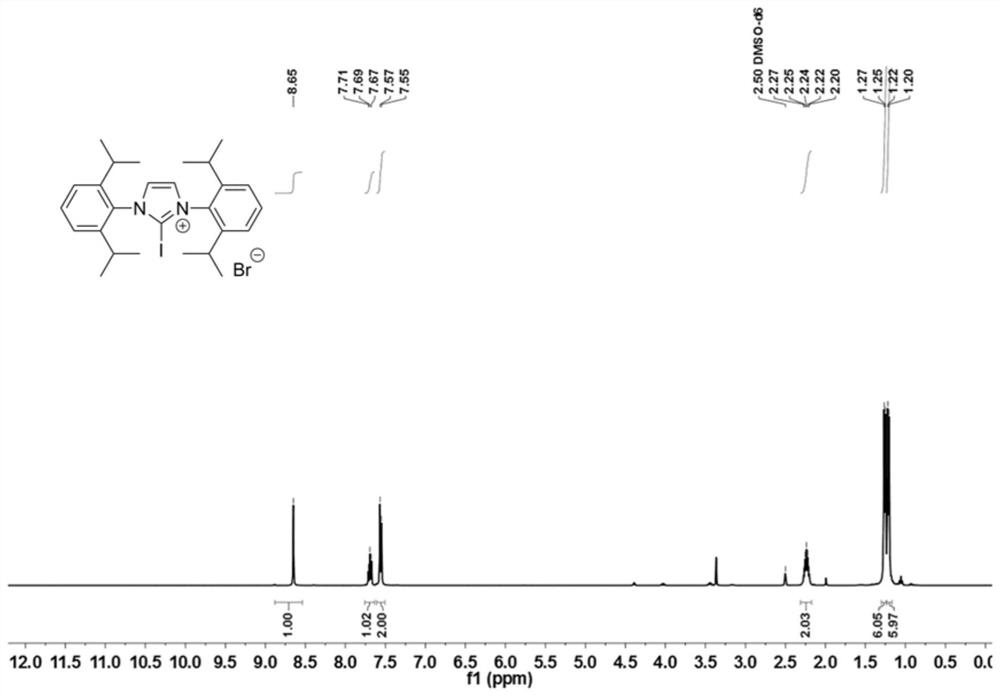
[0079] Dissolve catalyst 1 (0.55g, 1.0mmol, 1.0equiv.) in anhydrous dichloromethane, add sodium tetrafluoroborate (0.12g, 1.1mmol, 1.1equiv.) to form a suspension, and stir at room temperature for 2 hours, After filtration, the filtrate was spin-dried to obtain the tetrafluoroborate of Catalyst 1. Ethyl acetate was added to the dried tetrafluoroborate of Catalyst 1 (0.6 g, 1.0 mmol, 1.0 equiv.) to form a suspension. Configure a saturated solution of sodium iodide in acetone (0.6g, 4mmol, 4.0equiv.) and add the saturated solution dropwise to the above suspension, stir the mixed reaction solution at room temperature for 12 hours, filter, and dilute the filter residue with ethyl acetate The ester and a small amount of acetone were washed, and the filter residue was dried to obtain catalyst 2 as a white powder. 1 H NMR (400MHz, DMSO-d 6 )δ8.65(s, 2H), 7.69(t, J=7.8Hz, 2H), 7.56(d, J=7.8Hz, 4H), 2.24(p, J=6.7Hz, 6H), 1.24(dd, J=18.3,6.8Hz,12H). 13 C NMR (101MHz, DMSO-d 6 )δ144...
Embodiment 3
[0081] The 250mL reaction bottle was fully dried and filled with inert gas protection. Add 1,3-(2,6-diisopropylphenyl)-4,5-methylimidazolium chloride (4.53 g, 10 mmol, 1.0 equiv.), and add 100 mL of anhydrous dichloromethane to dissolve it , then added N-iodosuccinimide (2.36g, 10.5mmol, 1.05equiv.), stirred at 40°C for 2 hours, washed the reaction solution with deionized water 50×3, combined the organic phases, dried, and reduced The solvent was removed under pressure to obtain Catalyst 3 as a light yellow powder with a yield of 96%. 1 H NMR (400MHz, DMSO-d 6 )δ7.89–7.68 (m, 2H), 7.60 (d, J = 7.8Hz, 4H), 2.24 (p, J = 6.8Hz, 4H), 2.17 (s, 6H), 1.23 (dd, J = 6.8 ,2.9Hz,24H). 13 C NMR (101MHz, DMSO-d 6 )δ145.02, 132.45, 131.71, 129.84, 125.57, 107.02, 28.66, 23.60 (d, J=19.9Hz), 10.06.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap