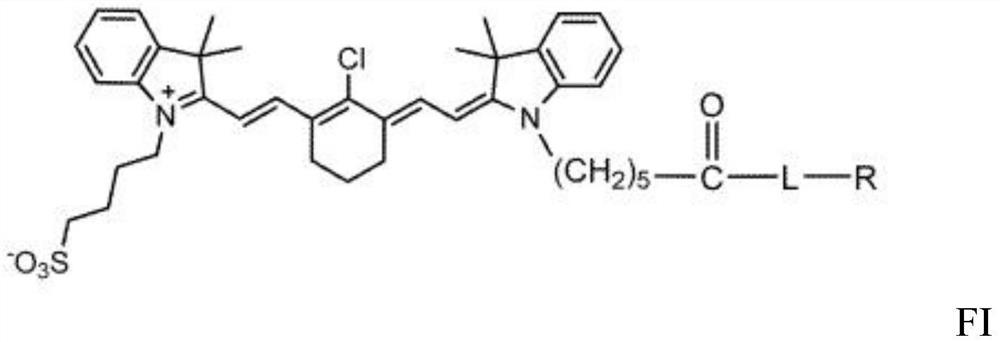
Heptamethyl carbonyanine dye-crosslinked tetracyclic amine chelating agent conjugate, and compound and application thereof
A technology of heptamethyl carbocyanine and chelating agent is applied in the field of heptamethyl carbocyanine dye-cross-linked tetracyclic amine chelating agent conjugate, and can solve the problems of unwanted radioactivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Example 1: Synthesis of DZ-1-(L)-CTC Chelator-Coupled Radiometal Complex
[0109] The synthesis of DZ-1 dye and its derivatives has been described previously, for example in US 10307489, which is hereby incorporated in its entirety, and can be performed as detailed in Example 1a below. The conjugate can then be complexed with a radiometal as described in Example 1b. The following chemicals and reagents can be used.
[0110] CTC chelators CB-TE2A and DiAmSar are commercially available from Macrocyclics (Plano, Texas). All other chemicals mentioned herein, can be purchased from various standard sources such as VWR International (Radnor, PA) or Thermo Fisher Scientific (Waltham, MA), as will be apparent to those of ordinary skill . Deionized ultrapure water (18.2 MQ) was used to prepare the solution, which is available from Milli-Q Direct Ultrapure Water Systems from Millipore Corporation (Billerica, MA, USA). Analytical Reverse Phase (RP) High Performance Liquid Chrom...
Embodiment 2
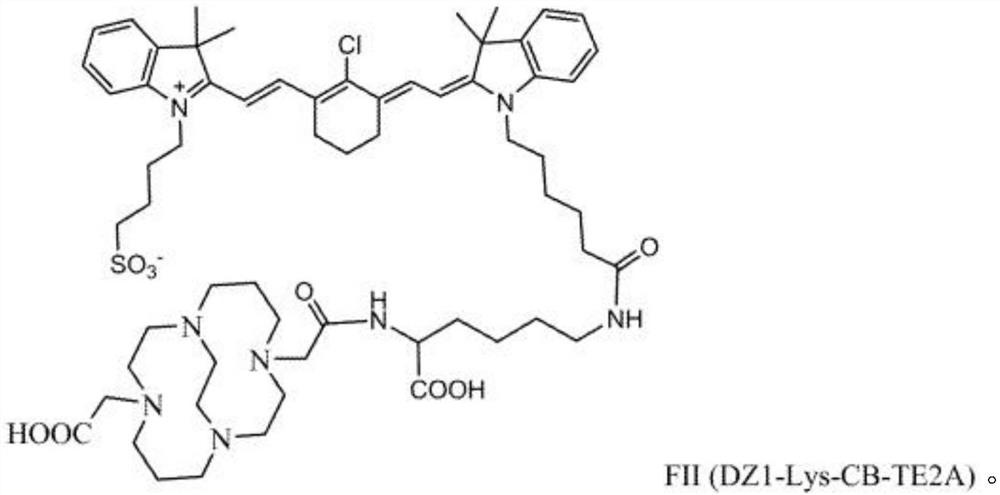
[0122] Embodiment 2: the synthesis of DZ-1-L-CTC conjugate
[0123] Scheme 3 below illustrates one option for the synthesis of DZ-1-L-CTC, here DZ-1-lysine-CB-TE2A8:
[0124]
[0125] Synthesis and radiolabelling of DZ-1-(L)-CTC, here DZ-1-lysine-CB-TE2A 8 (compare Scheme 3). An appropriate form of carbon tetrachloride (here CB-TE2A), such as one of its salts, such as hydrochloride, can be mixed with appropriate amounts of diisopropylcarbodiimide (DIC) and diisopropylethyl amine (DIEA), or triethylamine (Et 3N) mix. For example, CB-TE2A-4HCl salt 7 (50 mg, 0.11 mmol), diisopropylcarbodiimide (DIC) (14 mg, 0.11 mmol) and diisopropylethylamine (DIEA) (14 mg, 0.11 mmol ) is dissolved in an appropriate volume of DMF, for example 2ml of dimethylformamide (DMF). The mixture may be stirred for an appropriate amount of time to ensure complete dissolution and / or mixing, for example about 30 minutes. DZ-1-L (a conjugate with terminal aminocarboxyl group), such as DZ-1-lysine 6 (...
Embodiment 3
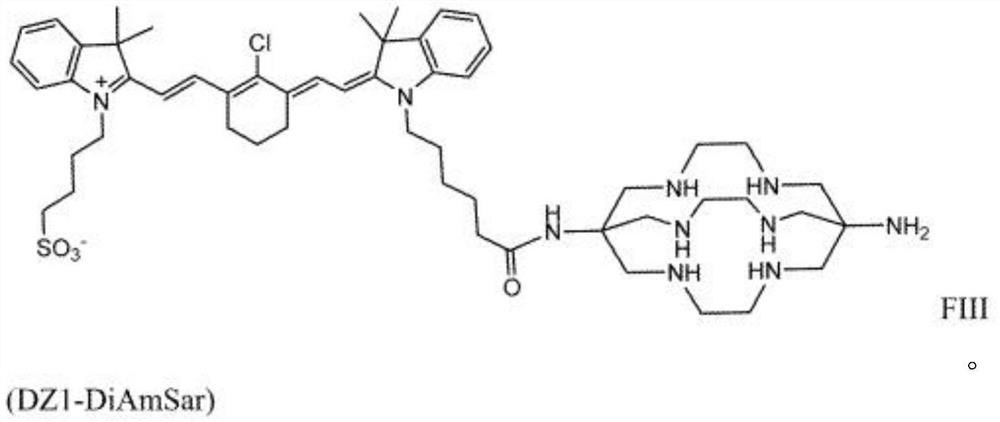
[0129] Embodiment 3: the synthesis of the DZ-1-CTC conjugate that does not contain cross-linking agent
[0130] Scheme 5 below illustrates the synthesis of DZ1-CTC without crosslinker, here DZ-1-DiAmSar:
[0131]
[0132] Synthesis of DZ-1-DiAmSar (comparison scheme 5): an appropriate amount of DZ-14 (for example about 50mg, 0.071mmol), diisopropylcarbodiimide (DIC) (for example about 13.5mg, 0.11mmol) and hydroxyl A mixture of benzotriazoles (HOBt) (eg about 11.5 mg, 0.085 mmol) was dissolved in an appropriate amount of DMF (eg about 2 ml DMF). The mixture was stirred well to completely dissolve and mix, for example about 30 minutes, then CTC (here DiAmSar 5H O 10 (29 mg, 0.071 mmol)) was added and stirred well to react, for example about 5 Hour. The product can be precipitated in an appropriate volume of medium, such as cold diethyl ether (e.g. about 40 ml), at a suitable low temperature. The precipitate is separated, e.g., centrifuged at a suitably high speed for a su...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com