Prophylactic or therapeutic agent and medicinal composition for il-31 mediated disease
A therapeutic agent and mediated technology, applied in the field of preventive or therapeutic agents and pharmaceutical compositions of IL-31 mediated diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0210] In one embodiment, the present invention provides a method for preventing or treating IL-31-mediated diseases, which includes the step of administering an effective amount of a neurokinin B signal blocker to a patient in need of treatment.
[0211] In one embodiment, the present invention provides neurokinin B signaling blockers for preventing or treating IL-31-mediated diseases.
[0212] In one embodiment, the present invention provides a neurokinin B signaling blocker for use in the manufacture of a prophylactic or therapeutic agent for IL-31-mediated diseases.
[0213] In each of the above embodiments, the neurokinin B signal blocker may be a neurokinin 3 receptor antagonist. The aforementioned neurokinin B signal blocking agent may be an inhibitor of tachykinin processing enzyme or a decomposition promoter of tachykinin processing enzyme. The aforementioned neurokinin B signal blocker may also be an expression inhibitor of neurokinin B (NKB), neurokinin 3 receptor,...
Embodiment
[0218] Hereinafter, although an Example demonstrates this invention, this invention is not limited to the following Example.
[0219] [Materials and methods]
[0220] (mouse)
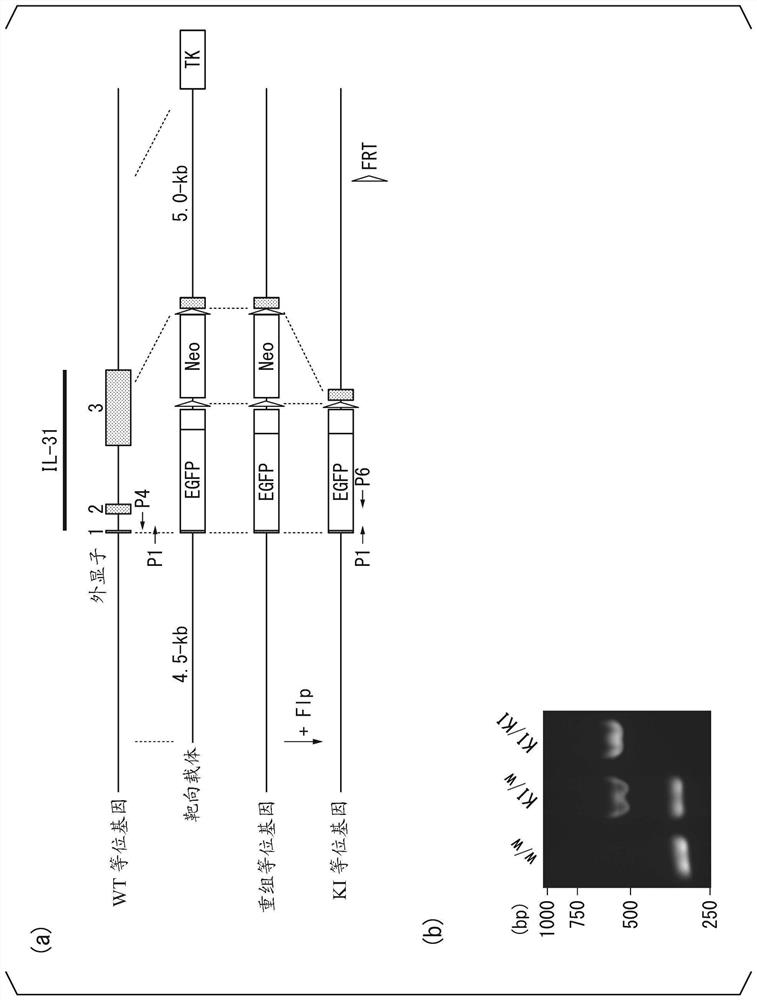
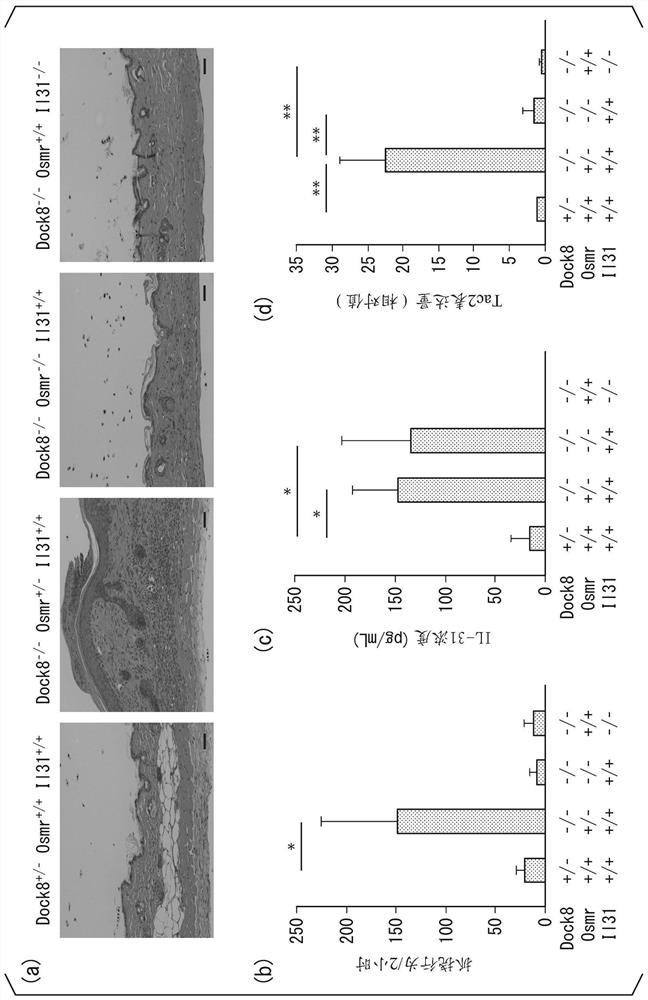
[0221] Tac2 - / - Mice were created by genome editing based on the CRISPR / Cas9 system. Target sites were selected within exon 4 of the mouse Tac2 gene using the CHOPCHOP web design tool (https: / / chopchop.rc.fas.harvard.edu / ). The oligonucleotides shown in SEQ ID NO: 3 and SEQ ID NO: 4 and the BbsI ligation adapter (LigationAdapter) were synthesized. The sequences shown in SEQ ID NO: 5 and SEQ ID NO: 6 are guide sequences. In order to co-express the sgRNA and the Cas9 protein, these two nucleotides were synthesized, annealed, and ligated into the px330 vector digested with BbsI. The prepared px330 vector (concentration 5ng / μL, Dulbecco's PBS (Dulbecco's phosphate buffer solution)) was injected into the pronuclei of C57BL / 6 mouse zygotes fertilized in vitro in M2 medium (sigma company) middle. Inject...
experiment example 1
[0238] (the neural circuit that transmits the itching sensation)
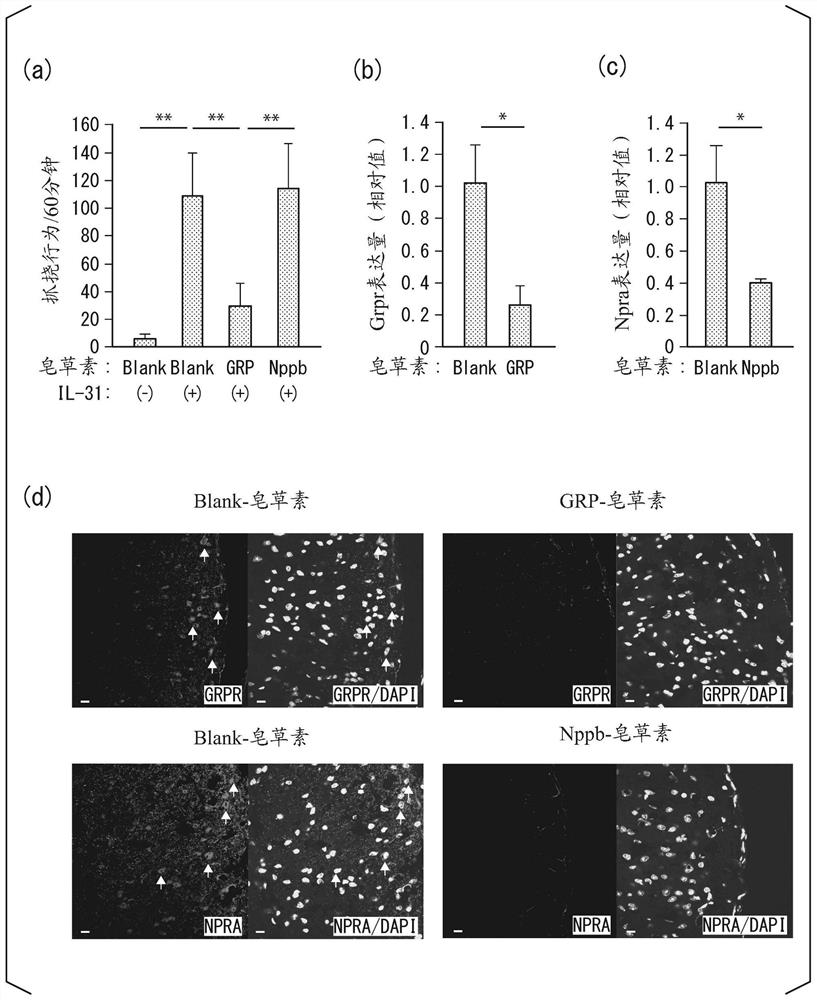
[0239] Neural circuits activated by IL-31 that convey the itch sensation were analyzed. Most itch-causing substances are mediated by natriuretic polypeptide b (Nppb) or gastrin-releasing peptide (GRP) in spinal nerves to transmit itching sensation.
[0240] In order to remove nerve cells expressing GRP receptors or Nppb receptors, wild-type C57BL / 6 mice were injected intramedullary with saporin-conjugated GRP or Nppb (GRP-saporin, Nppb-saporin, respectively). 2 weeks later, IL-31 was administered to these mice, and the scratching behavior of each mouse was analyzed.
[0241] Saporin is a kind of ribosome-inactivating protein (RIP).
[0242] The protein conjugated to the receptor ligand expressed in the target nerve cell and RIP is captured by the target nerve cell and swallowed into the cell through endocytosis. The engulfed protein conjugated to RIP inhibits translation and kills target neuronal cells.
[...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com