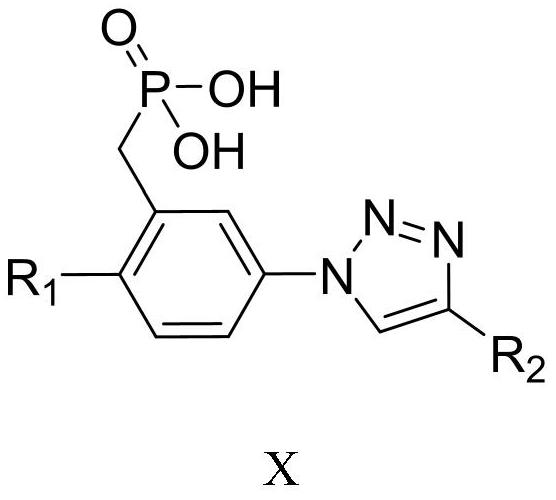
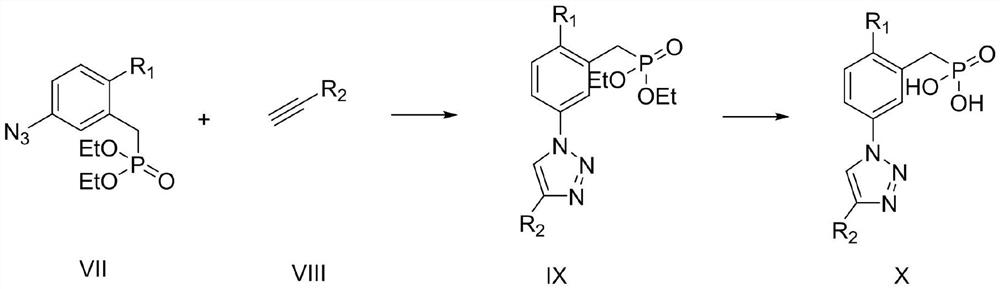
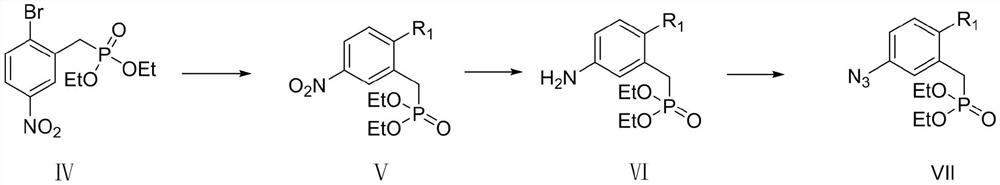
(3-(1H-1,2,3-triazole)phenyl)phosphoric acid derivative as well as preparation method and application thereof
A technology of phosphoric acid and derivatives, which is applied in the field of phenyl) phosphoric acid derivatives and their preparation, can solve problems such as insufficient bacterial resistance, and achieve excellent antibacterial activity and broad-spectrum inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0050] The raw materials and equipment used in the specific embodiment of the present invention are all known products, obtained by purchasing commercially available products.
[0051] 1. the preparation method of intermediate compound IV, comprises the following steps:
[0052]
[0053] Step 1: Dissolve 2-bromo-5-nitrobenzaldehyde 1 (2g, 8.69mmol) in 30ml ethanol, add sodium borohydride (166, 4.36mmol) three times at room temperature, react for 20 minutes, and monitor with TCL The degree of progress of the reaction, after the complete reaction, add 10ml of water to quench, then add 10ml of dichloromethane, shake the separatory funnel up and down for 1 minute, let stand to separate layers, collect the lower organic phase, repeat this process three times, combine the organic phases, and dry After drying over sodium sulfate, the solvent was concentrated and removed to obtain a crude product, which was obtained by column chromatography as a light yellow solid compound II (2-br...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com