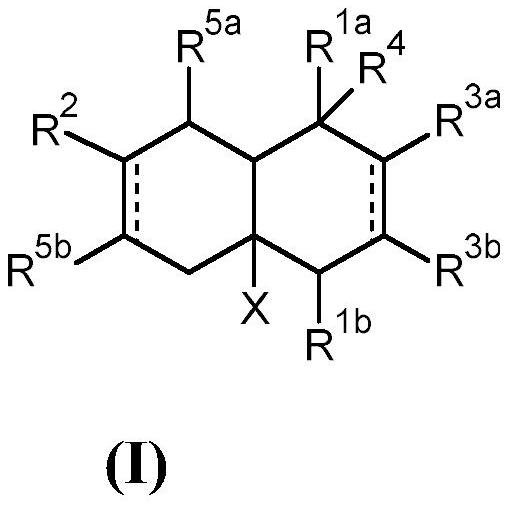
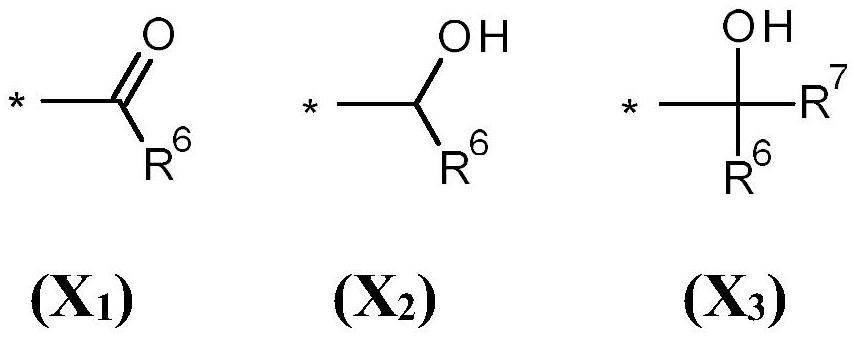
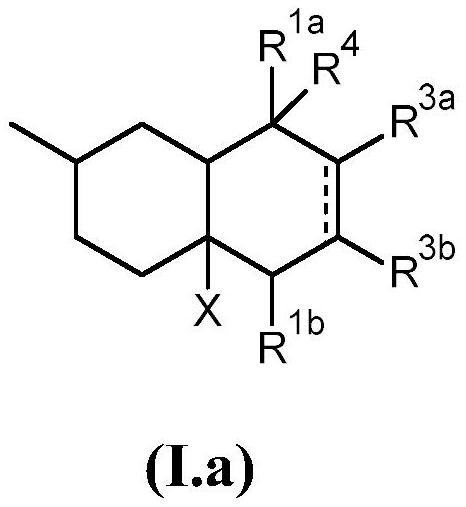
Bi- and tricyclic compounds for use as aroma chemicals
A technology of compounds, mixtures, applied to bicyclic and tricyclic ketones, to prepare polymerizable compounds, to prepare said compounds, with the aim, in the field of fragrances, to be able to solve substances, organoleptic properties that do not know whether odor and/or taste are actually found Changes, difficulty finding new flavors and seasonings, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0363] abbreviation
[0364] GC: gas chromatography
[0365] MTBE: methyl tert-butyl ether
[0366] EtOAc: ethyl acetate
[0367] analyze:
[0368] Through GC, 1 H-NMR (CDCl 3 ,500MHz) and / or 13 C-NMR (125MHz, CDCl 3 ) to determine the purity and identity of the product.
[0369] 1. Preparation Example
[0370] 1.1 Preparation of 1-((2R)-2,6,7-trimethyl-1,3,4,5,8,8a-hexahydronaphthalene-4a(2H)-yl)ethan-1-one:
[0371] In a 500 mL three-necked round bottom flask equipped with a thermometer and a 250 mL dropping funnel, combine 15 g (108.5 mmol) of 1-[(4R)-4-methylcyclohexen-1-yl]ethanone with AlCl 3 (1.5g) and toluene (200mL). A solution of 2,3-dimethyl-1,3-butadiene (18.2 g, 221.6 mmol) in toluene (100 mL) was added slowly via the dropping funnel over 4 hours. The reaction was stirred at room temperature for an additional 48 hours. Water (100 mL) was added to the reaction mixture to quench the reaction, and acetic acid (50%) was added until all gel-like materi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com