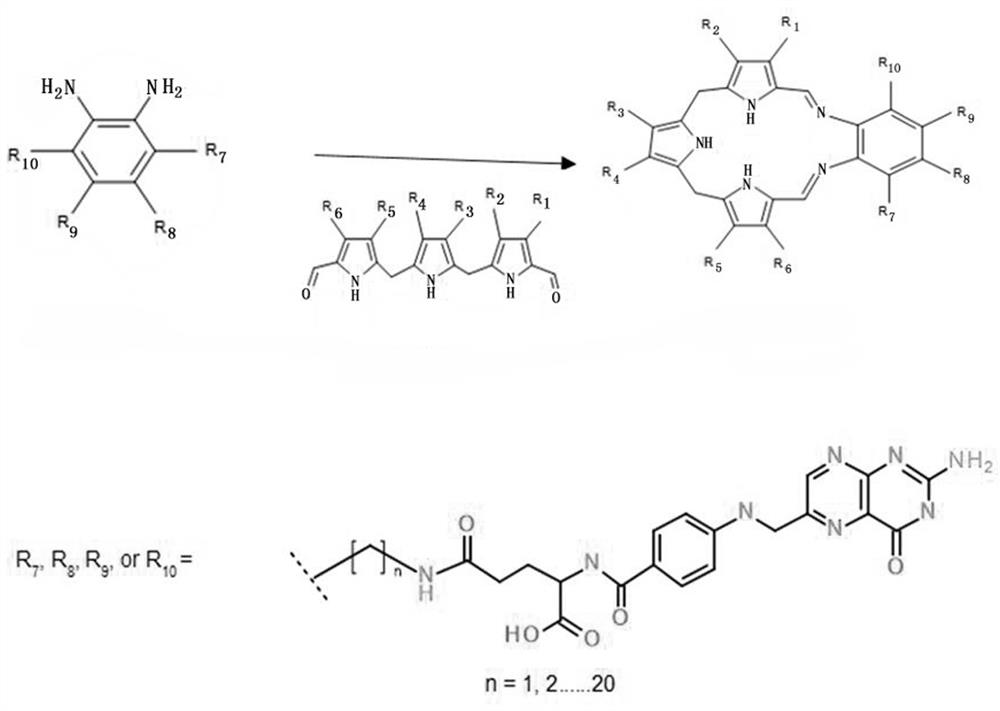
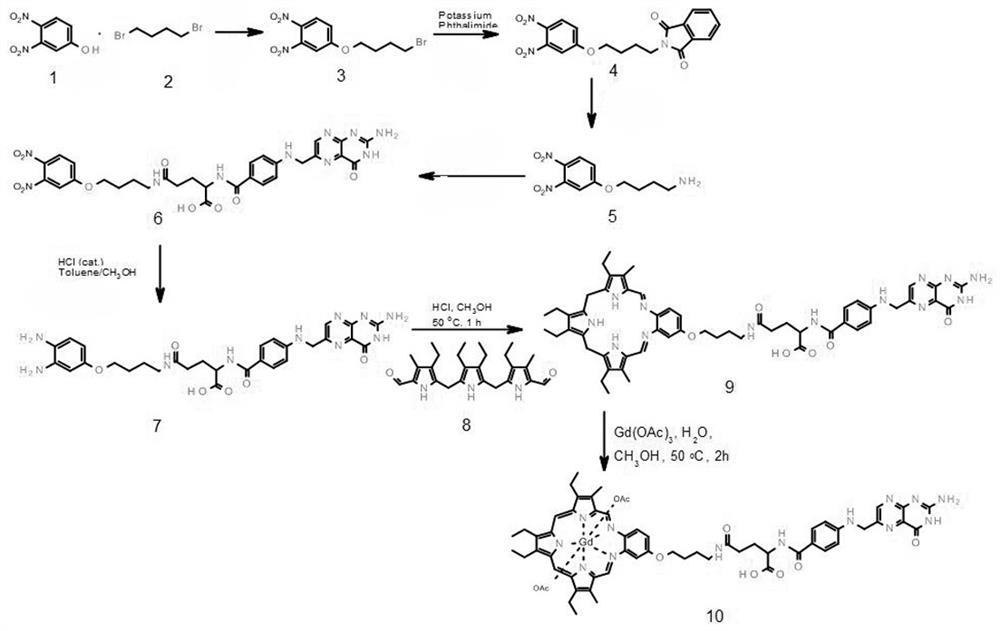
Texaphyrin-folic acid conjugate as well as preparation method and application thereof
A technology of chelate and folic acid derivatives, which can be used in drug combinations, pharmaceutical formulations, radioactive preparations in vivo, etc., and can solve the problem that there is no Texaphyrin-folate chelate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0093]Orthotopic PC3 prostate tumor model: Athymic nude male mice were purchased from Harlan Laboratories. A 5 mm long incision into the peritoneum was made in the abdomen; the bladder and seminal vesicles were partially excised from the abdominal cavity to expose the dorsal lobe of the prostate. Inject 5×105 PC-3M-luc-C6 cells (cell line expressing Lucifer, Caliper)
[0094] Use a 28-gauge needle from in situ to the dorsal lobe of the prostate. The organs were returned to the abdominal cavity, and the muscle wall and skin were sutured. Subcutaneous injection of 0.05 mg kg-1 for analgesia. Orthotopic prostate tumor growth was monitored by MRI (Biospec 70 / 30 USR, Bruker).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com