Methods for allogeneic hematopoietic stem cell transplantation
A hematopoietic stem cell, allogeneic technology, applied in blood/immune system cells, animal cells, extracellular fluid diseases, etc., can solve problems such as reducing the efficacy of transferred cells and restricting the use of allogeneic cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0755] Preparation of antibody-drug conjugates
[0756] In the ADC of Formula I as disclosed herein, an anti-HC antibody (such as an anti-CD117 antibody or an anti-CD45 antibody) or an antigen-binding fragment thereof is linked to one or more cytotoxic drug moieties (D ) conjugated, for example, from about 1 to about 20 drug moieties per antibody. The ADCs of the present disclosure can be prepared in a variety of ways, using organic chemical reactions, conditions and reagents known to those skilled in the art, including: (1) Reactive substituents of antibodies or antigen-binding fragments thereof react with divalent linking reagents to form Ab-Z-L as described above, subsequently reacted with the drug moiety D; or (2) the reactive substituent of the drug moiety is reacted with a divalent linking reagent to form D-L-Z', subsequently reacted with the antibody or its antigen as described above The reactive substituents of the bound fragment react to form an ADC of formula D-L-...
Embodiment 1
[0766] Example 1: Anti-CD45 and anti-CD117 antibody drug conjugates realize allogeneic hematopoietic stem cell transplantation
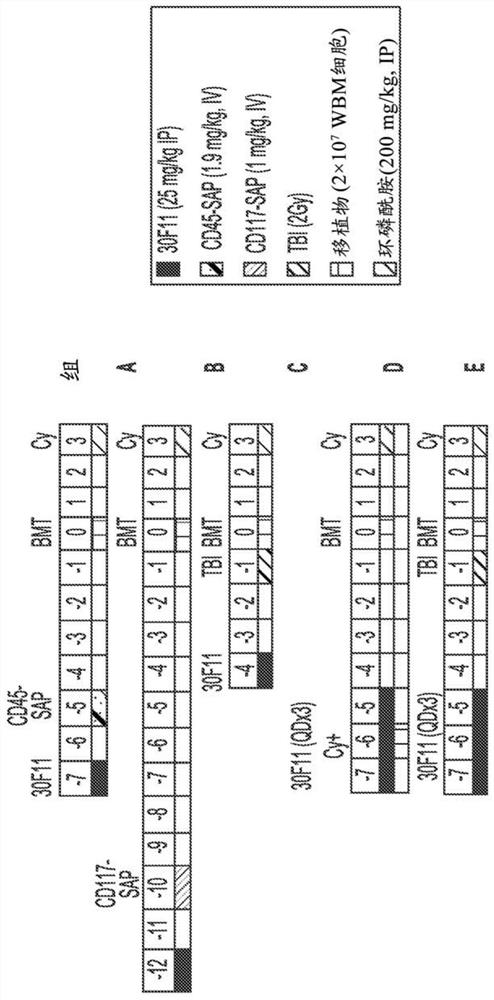
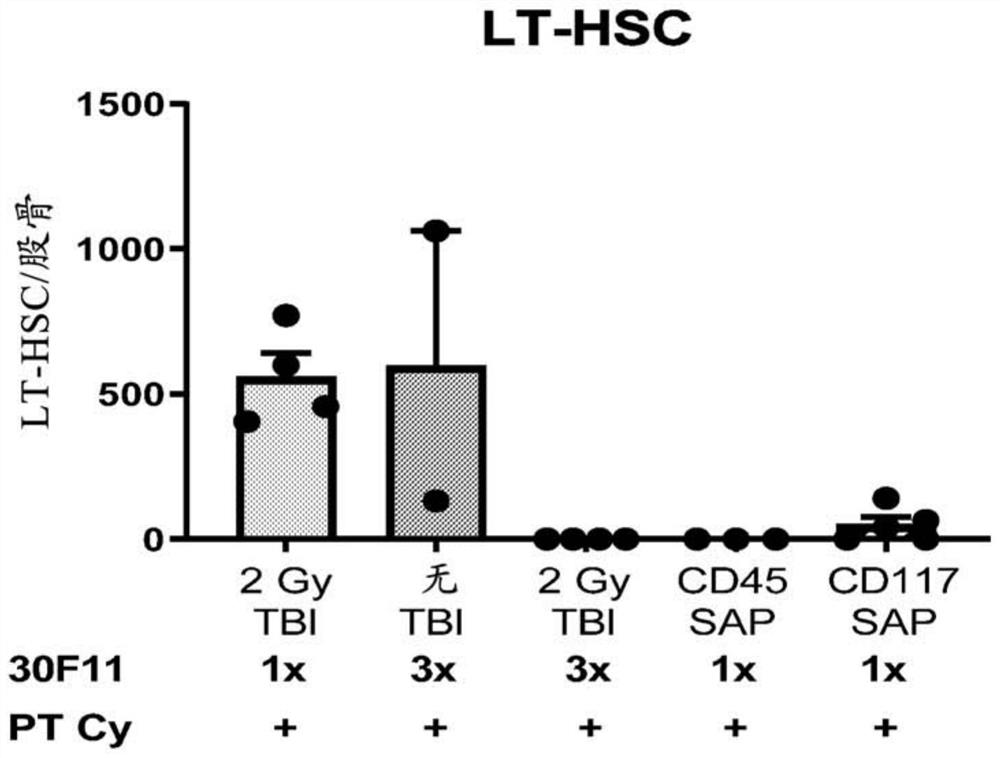
[0767] Antibody drug conjugates (ADCs) targeting mouse CD45 or mouse CD117 were recently shown to effectively opsonize immunocompetent mice for whole bone marrow transplantation (Palchaudhuri et al. Nature Biotech 2016 34:738–745; and Czechowicz et al. al. Blood 2016 128:493). This innovative targeting approach utilizing ADCs for conditioning has the potential to be a therapeutic breakthrough if successfully applied to humans. Previously used anti-CD45 or anti-CD117 antibodies were conjugated to saporin, a ribosomal inhibitory protein, which, once internalized, causes cytotoxicity in a cell cycle-independent manner. Both anti-CD45-saporin (CD45-SAP) and anti-CD 117-saporin (CD117-SAP) ADCs have been shown to effectively deplete bone marrow hematopoietic stem cells (HSCs) as single-entity agents, thereby creating a gap that achieves effective autolo...
Embodiment 2
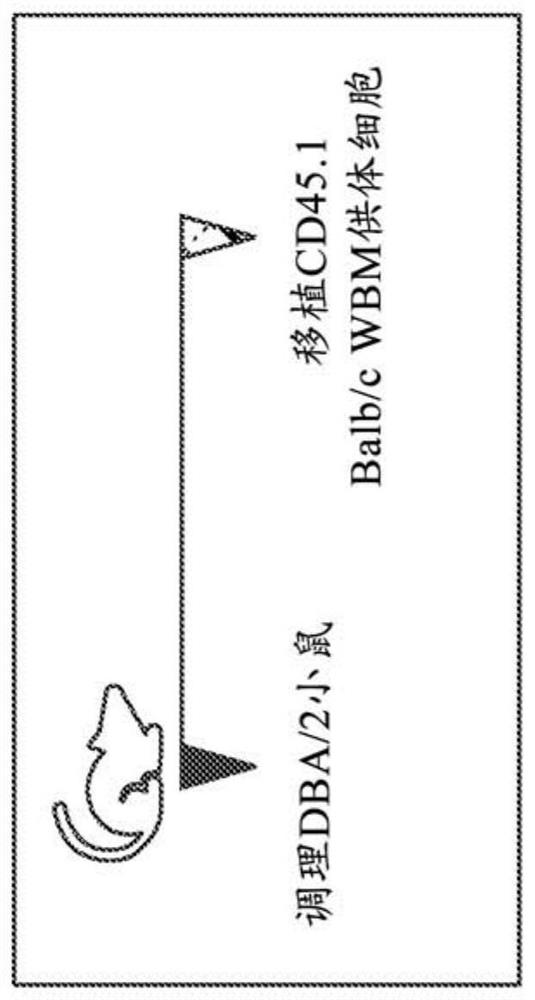
[0772] Example 2: CD45-Targeting Antibody Drug Conjugate Plus Post-Transplant Cyclophosphamide Sufficient to Achieve Allogeneic Bone Marrow Transplantation in a Minor Mismatch Mouse Model
[0773] Bone marrow transplantation (BMT) is a potentially curative treatment for malignant and non-malignant blood disorders. Current patient preparation or conditioning protocols prior to BMT limit the use of this curative procedure due to protocol-related mortality and morbidity, including organ toxicity, infertility / infertility, and risk of secondary malignancies. It was previously shown that targeted preparation using an antibody-drug conjugate (ADC) against mouse CD45 is sufficient to achieve bone marrow transplantation (BMT) in immunocompetent syngeneic mice (Palchaudhuri et al. Nature Biotech 2016 34:738– 745), and this preparation, if successfully applied to patients, has the potential to expand the utility of BMT. To further investigate the utility of anti-CD45 ADC (anti-CD45 anti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com