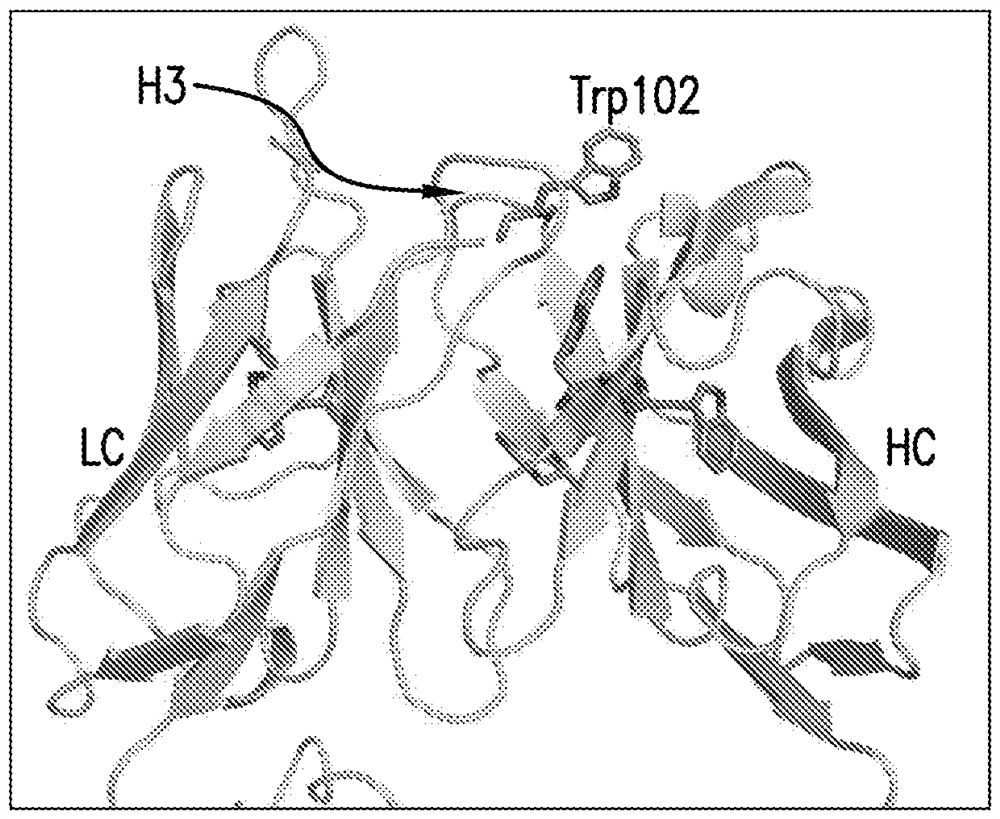
Co-formulations of Anti-lag3 antibodies and Anti-pd-1 antibodies
A PD-1, preparation technology, applied in the field of therapeutic antibody preparation, can solve problems such as impaired immune response, weakened T cell activation and immune surveillance evasion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
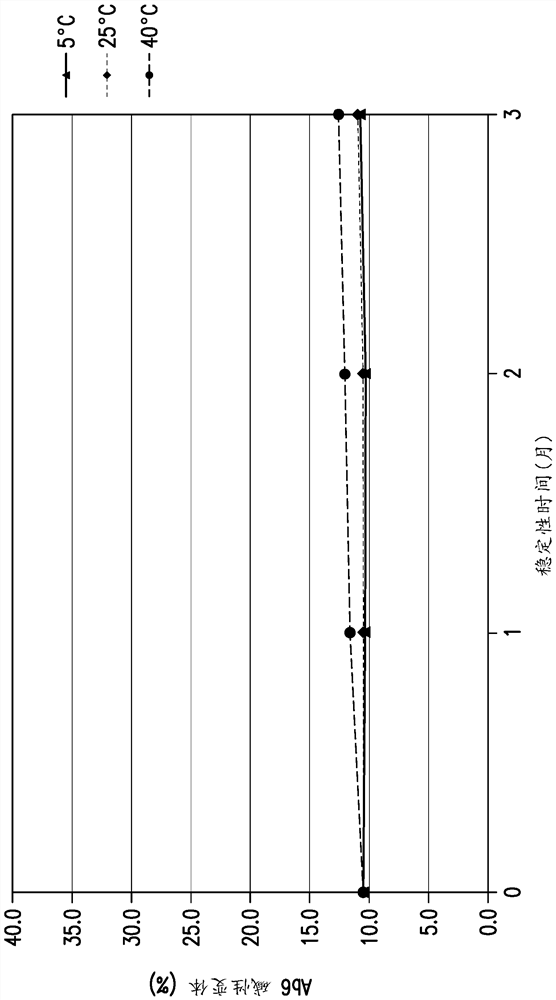
[0341] Example 1: Study of Conditions to Reduce Self-Association of Anti-LAG3 Antibody Ab6
[0342] Diffusion interaction parameter (k D )Measurement
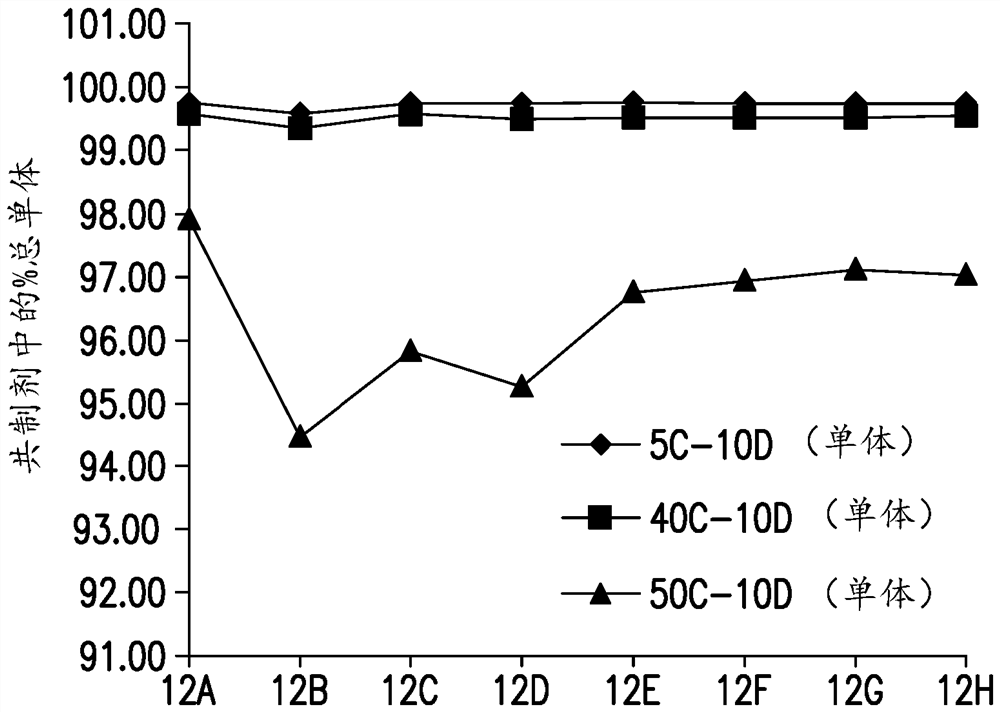
[0343] B found in 10 mM histidine pH 5.6 22 is negative, which indicates the intrinsic property of molecular self-association. It was found that the presence of 50 mM sodium chloride in 10 mM histidine pH 5.6 increased the diffusion interaction parameter (KD) of anti-LAG3 antibody Ab6 (SEQ ID NO: 35 and 57, light chain and heavy chain) or decreased its self-interaction, Improved its relative solubility and reduced turbidity (OD350), such as Figure 12 shown. In the presence of 40 mM L-arginine hydrochloride, the anti-LAG3 antibody was studied at 10 mM histidine pH using diffusion interaction parameters (KD), turbidity (OD350) and relative solubility (% PEG midpoint) assays. Stability in 5.6. Such as Figure 13 As shown, the self-interaction and turbidity were found to be drastically reduced, while the relative solubi...
Embodiment 2
[0367] Example 2: Preformulation Screening with Charged Species (Salts and Amino Acids) of Anti-LAG3 Antibody Ab6 Formulations
[0368] To evaluate the stability of anti-LAG3 antibodies in the presence of charged species (salts and amino acids), ten formulations listed in Table 5 were prepared and screened for the physicochemical properties of anti-LAG3 antibodies by high-throughput analysis The change. Formulations were properly sealed in 96-well plates and stressed in a dry heat oven at 50 °C for 10 days. Changes in the physicochemical properties of the anti-LAG3 antibodies were also evaluated for heat-stressed samples. A 20 mM concentration of L-aspartic acid or L-glutamic acid was chosen based on its solubility limit.
[0369]
[0370] Turbidity (OD 350 ) scheme
[0371] The turbidity (OD) of nine preparations was evaluated using an ultraviolet (UV) absorption spectrophotometer 350 ). UV absorbance of samples was measured at a wavelength of 350 nm in a 96-well...
Embodiment 3
[0383] Example 3: Screening of Stabilizers for Anti-LAG3 Antibody Ab6 Preparations
[0384] To evaluate the anti-LAG3 antibody Ab6 (25 mg / mL in 10 mM L-histidine, 70 mM L-arginine hydrochloride or in 70 mM sodium chloride) in the presence of different stabilizers such as sugars and polyols , pH 5.8), prepared eleven formulations as listed in Table 6.
[0385]
[0386] Ultra Performance Size Exclusion Chromatography (UP-SEC)
[0387] The purity of the samples was assessed by UP-SEC, in which the percentage of monomer was determined, as well as the percentage of high molecular weight species (HMW) and late eluting peaks (LMW species). UP-SEC was performed on a Waters Acquity UPLC system H class Bio by diluting the sample to 1.0 mg / mL in mobile phase (100 mM phosphate, 100 mM sodium chloride, pH 7.0). The column temperature was maintained at 25±3°C, and the flow rate was maintained at 0.5 mL / min, using isocratic elution. Diluted samples (5 μL) were injected into a UPLC eq...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| osmolarity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com