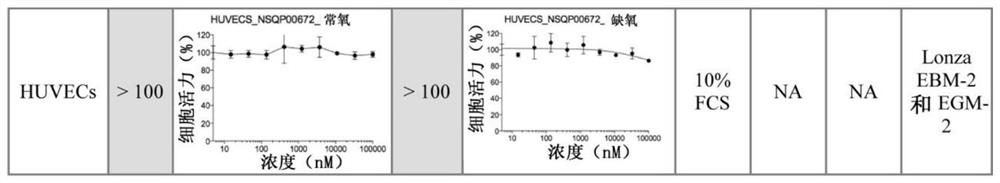
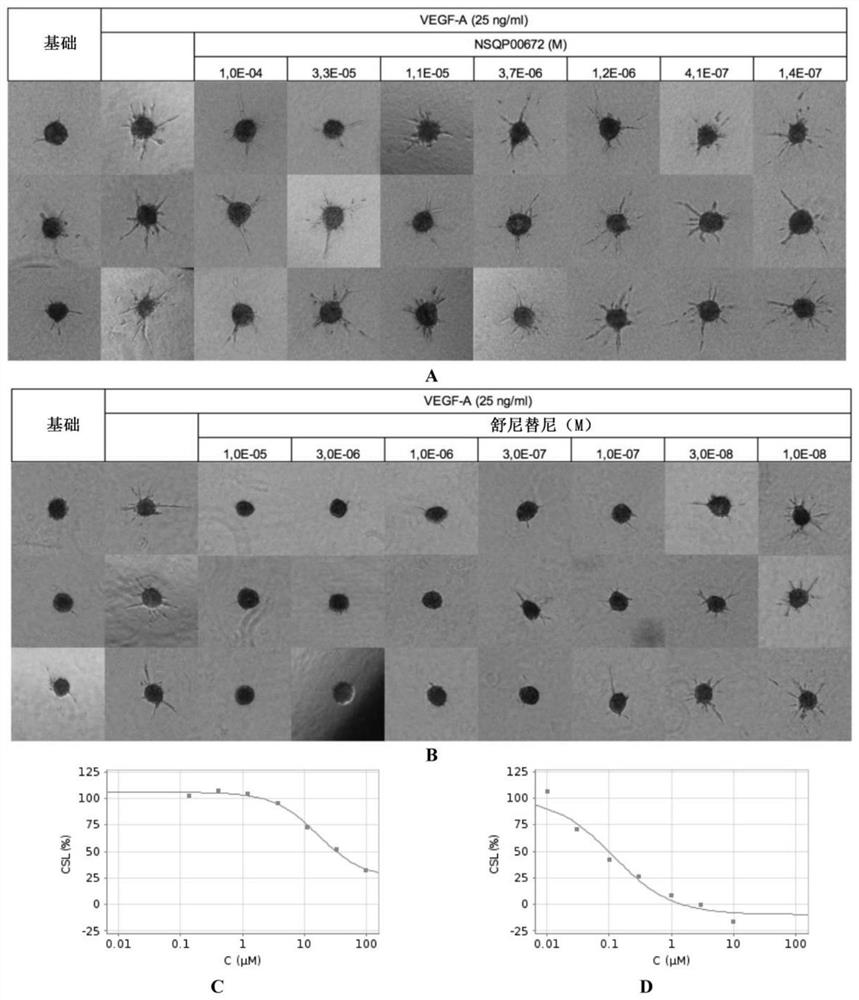
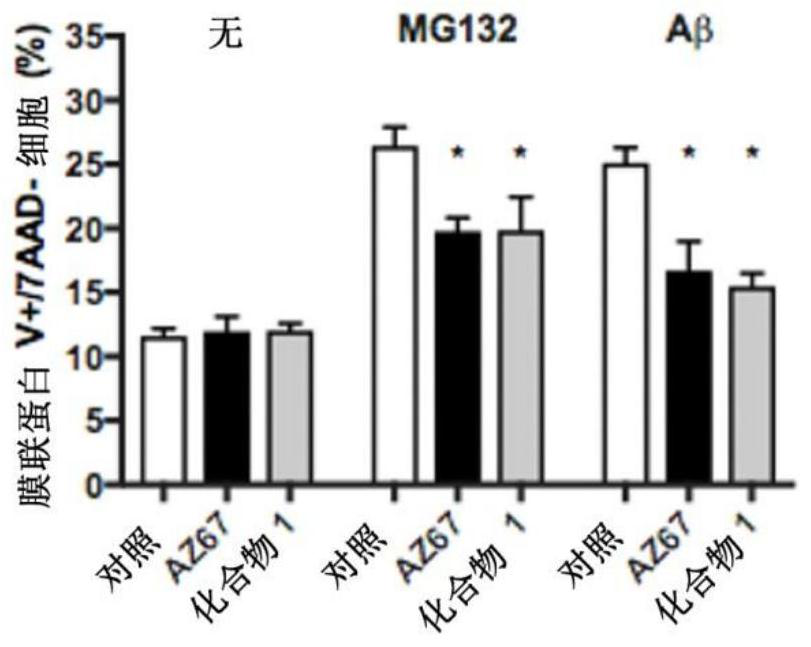
Pfkfb3 inhibitors and their uses
A C3-C8, -O-C3-C8 technology, applied in the field of PFKFB3 inhibitors and their uses, can solve problems such as reduction and unclear inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1203] Compound preparation
[1204] The compounds used in the reactions described herein are prepared according to known organic synthesis techniques starting from commercially available chemicals and / or compounds described in the chemical literature. "Commercially available chemicals" are obtained from standard commercial sources. The following non-exhaustive and non-exclusive commercial list of commercial suppliers is provided by way of example and reference only, and compounds useful in the present invention may be obtained from other suppliers: Acros Organics (Gill, Belgium), Aldrich Chemical (Milwaukee, WI, including Acros Organics ( Gill, Belgium), Aldrich Chemical (Milwaukee, Wisconsin including SigmaChemical and Fluka), Alfa Aesar (Heysham, UK), Alfa Chemistry (Holtsville, New York), Angene International Limited (London, UK), Apin Chemicals Ltd. (Milton, UK (Milton Park)), Apollo Scientific Ltd (Stockport, UK), Ark Pharm, Inc. (Libertyville, Israel), AuroraFine Chemi...
Embodiment 1
[1859] Example 1: 2-(2-Methoxybiphenyl-4-yl)-1,3-dioxo-2,3-dihydro-1H-isoindole-5-carboxylic acid
[1860]
[1861] 129. 2-(2-Methoxybiphenyl-4-yl)-1,3-dioxo-2,3-dihydro-1H-isoindole-5-carboxylic acid was synthesized as described in Synthetic Process K Preparation from trimellitic anhydride and 2-methoxy-[1,1'-biphenyl]-4-amine; MS m / z: (M+H) + for C 22 h 15 NO 5 Calculated: 374.36; detected: 374.14. LC / MS retention time: 2.53 minutes.
Embodiment 2
[1862] Example 2: 1,3-dioxo-2-[3-(1H-tetrazol-5-yl)-1,1'-biphenyl-4-yl]-2,3-dihydro-1H- Isoindole-5-carboxylic acid
[1863]
[1864] 130. 1,3-Dioxo-2-[3-(1H-tetrazol-5-yl)-1,1'-biphenyl-4-yl]-2,3-dihydro-1H-isoindol Indole-5-carboxylic acid was prepared from trimellitic anhydride and crude 3-(1H-tetrazol-5-yl)[1,1'-biphenyl]-4-amine (interm. 18) as described in Synthetic Step K; MS m / z:(M+H) + for C 22 h 13 N 5 o 4 Calculated value: 412.38; detected value: 412.26. LC / MS retention time: 2.19 minutes.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com