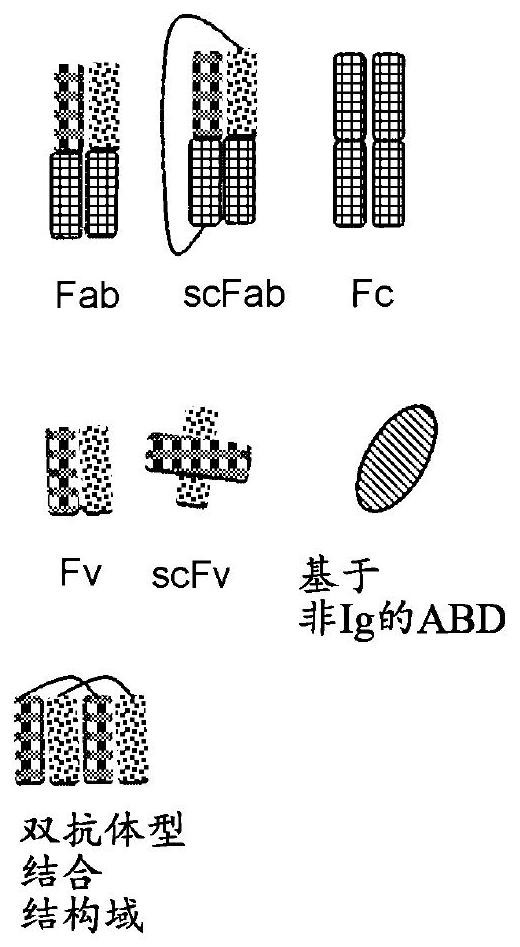
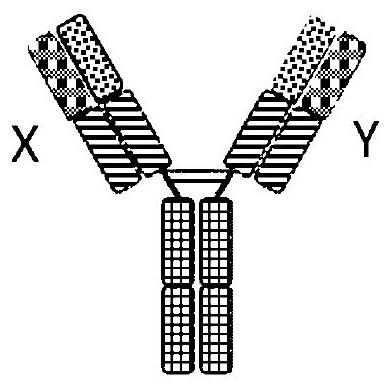
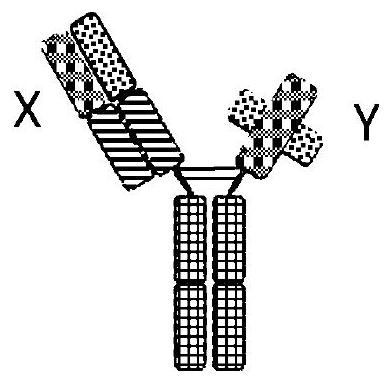Binding molecules against cd3 and uses thereof
A technology for binding molecules, CDR-L3, applied in the field of binding molecules for CD3 and their uses, which can solve the problems of antigen loss, biodistribution, inhibitory microenvironment, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[1041] 8.1. Example 1: Generation of anti-CD3 antibodies
[1042] Three to twenty week old rats were immunized with recombinant human CD3 protein or peptide by repeating the procedure involving four subcutaneous or intraperitoneal injections. The spleens of the immunized rats were harvested, and the isolated splenocytes were fused with myeloma cells (P3Ag8.653 cell line) to form hybridoma clones. with Mirrorball TM (TTPlabtech) tested supernatants of hybridoma clones as the primary screening assay to identify positive clones that bind to human CD3. The supernatants of positive clones identified from the primary screening binding assay against human CD3 were then tested for their ability to bind cynomolgus CD3. Only clones capable of binding both human CD3 and cynomolgus CD3 are provided.
[1043] Methods of primatizing or humanizing non-human antibodies are well known in the art. Typically, a primatized or humanized antibody has one or more amino acid residues introduced f...
example 2
[1071] 8.2. Example 2: Binding of CD-3 antibodies
[1072] The binding affinity interaction (KD) of anti-CD3 antibodies was determined using surface plasmon resonance (SPR) technology. CD3 protein was immobilized on an S-series CM5 sensor chip and antibodies were flown in 2-fold serial dilutions to assess binding using a Biacore T200 instrument (GE Heathcare, cat. no. 28975001, Pittsburgh, PA). KD was determined by fitting a curve with a 1:1 fitting model (O'Shannessy et al. Anal. Biochem 1993;212:457-468;Karlsson, J. Immunol. Methods. [J. Immunol. Methods] 1997;200:121-133). This SPR data is shown in Figures 4A-4D middle. The figure shows NOV292 ( Figure 4A ) and NOV123 ( Figure 4C ) is an effective binder, and with Figure 4B The SP34 positive control in is comparable. Figure 4D The SP1C antibody is a parental rat antibody that has not been humanized.
[1073] The binding activity (EC50) of anti-CD3 antibodies to human CD3 or cynomolgus CD3 stably expressed in C...
example 3
[1074] 8.3. Example 3: Activation of JNL cells by anti-CD3 agonist antibodies
[1075] Jurkat NFAT luciferase (JNL) cells were cultured in RPMI1640 medium supplemented with 10% FBS, 2 mM Glutamax (Gebroco, Cat. No. 35050061) and 0.5 μg / ml puromycin (Gebro, Cat. No. A1113802). . Plates were coated with serially diluted antibodies and incubated at 37°C for 1 hour. After incubation, the plates were washed with 200 μl of wash buffer (PBS+1% FBS), followed by 100 μl of prepared JNL cells (2.5x10 5 cells / mL) were added to all wells. Cells were incubated for 14h-16h at 37°C, 5% CO2. The plate was removed from the incubator and 100 μl of One-glo reagent (Promega, cat. no. E6120) was added. The reagents were incubated at room temperature for 3 minutes and then placed in a light sealed dark box for 10 minutes at room temperature. Samples were transferred to white solid bottom tissue culture plates and data were analyzed using an Envision plate reader (Perkin Elmer (Perkin Elmer, Ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com