A kind of m1 macrophage exosome vaccine and its preparation method and application
A technology of macrophages and exosomes, which is applied in the field of tumor immunotherapy drugs, can solve problems such as toxic side effects, high immunogenicity, and inability to effectively activate immune responses, so as to reduce toxic side effects, simple preparation method, and alleviate immunosuppression The effect of the phenomenon
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
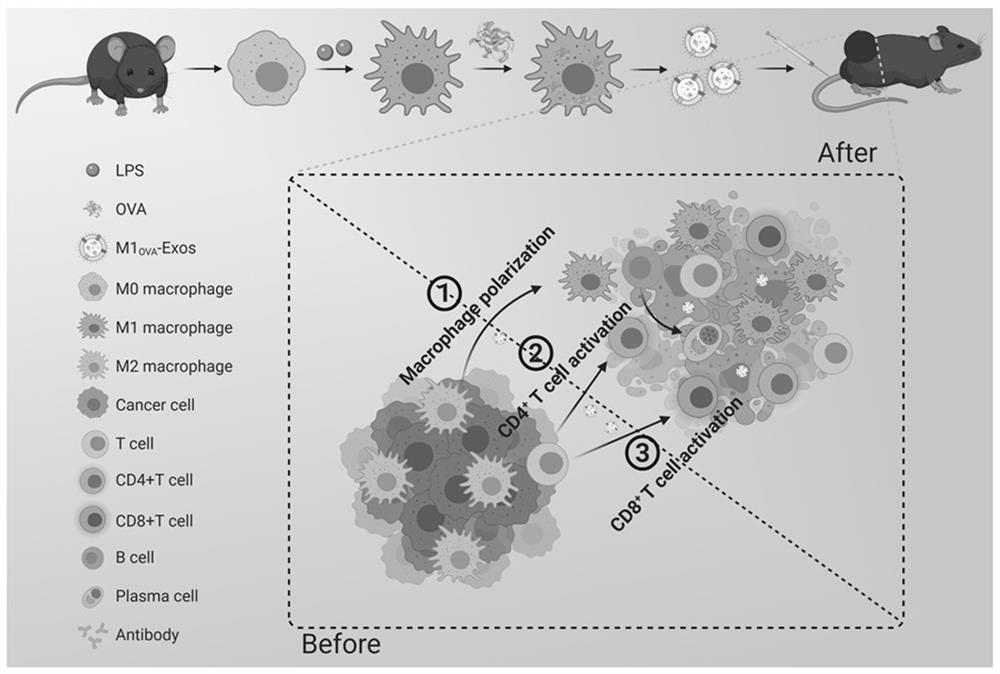
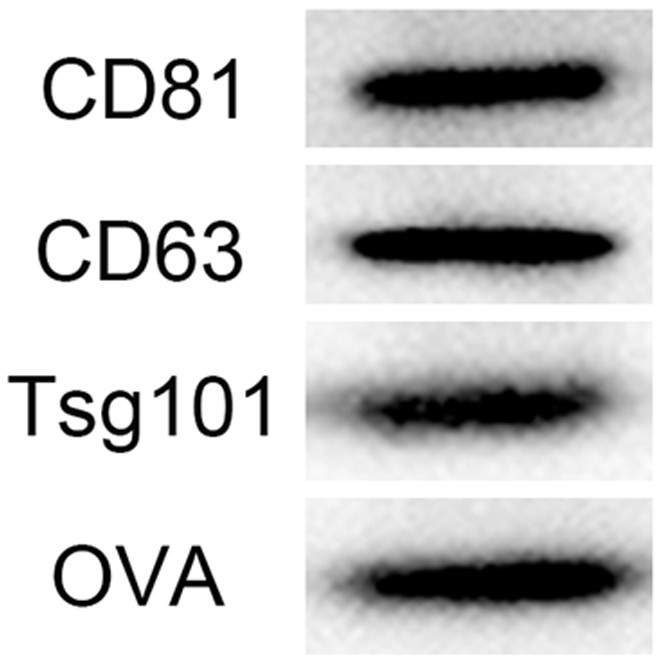
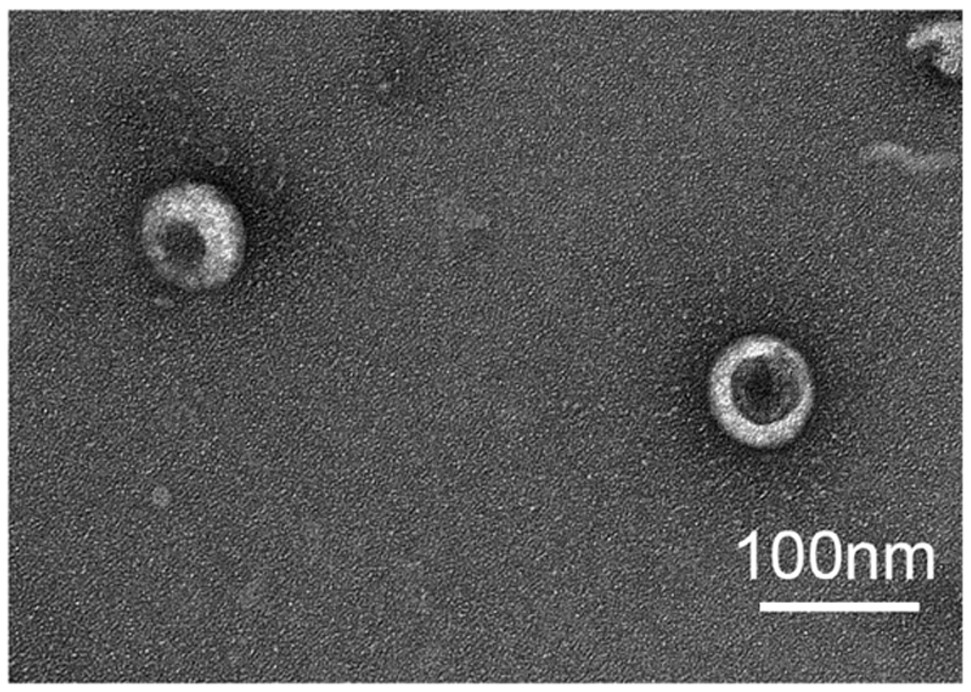
[0035] The following takes a widely used antigen model OVA as an example to introduce M1 in detail. OVA -Preparation method of Exos, such as figure 1 shown, including the following steps:
[0036] (1) Preparation of M1 macrophages carrying OVA
[0037] Female C57BL / 6 mice aged 6-10 weeks were selected, fasted the day before sacrifice, and starved for at least 8 h. After soaking and disinfecting with 75% alcohol, the operation is performed on the ultra-clean bench, and all items used must be clean and sterile. Fix the mouse to the foam board, lift the mouse abdominal fur with tweezers and cut open with scissors, do not cut the endothelium. Then lift the endothelium and cut a small mouth, hold it with tweezers, pour cold DMEM complete medium into the abdominal cavity, blow and suck a few times in the abdominal cavity, and gently stroke the abdomen of the mouse with fingers to fully lavage, collect the peritoneal lavage fluid, and repeat several times. times, centrifuged (100...
Embodiment 2
[0042] (1) M1 OVA -Exos in vitro immune activation ability evaluation
[0043] A large number of TAMs are present in the tumor microenvironment, and most of them exhibit an immunosuppressive M2 phenotype affected by the tumor microenvironment, which is a major obstacle to immunotherapy. We therefore adopted M1-Exos polarized macrophages as the M1 phenotype. like Figure 5 As shown, the results of the confocal laser show that M1-Exos and M1 OVA -Exos can increase the expression of CD80 and decrease the expression of CD206 on the surface of RAW264.7. This shows that M1-Exos is the same as M1 OVA -Exos can efficiently polarize macrophages to M1 phenotype. Image 6 Materials were analyzed for in vitro T cell activation capacity by flow cytometry. T cell Jurkat (clone E6-1) with PBS, OVA, M0-Exos, M1-Exos and M1 respectively OVA After co-incubation with -Exos, the cells were resuspended in PBS by centrifugation and CD4+ T cells and CD8+ T cells were labeled with fluorescent ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com