Tumor vaccine combining exosome with immune checkpoint blocker and preparation method thereof
A technology of immune checkpoints and tumor vaccines, applied in the field of tumor vaccines combined with immune checkpoint blockers and its preparation, can solve the problems of unsatisfactory therapeutic effects of tumor vaccines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Taking OVA, a widely used antigen model, as an example, the preparation method of Exo-OVA-aPD-L1 is described in detail below, such as figure 1 shown, including the following steps:
[0041] (1) Culture primary dendritic cells (DC)
[0042] After the C57BL / 6 female mice were killed by neck dislocation, they were soaked in 75% alcohol for 5-10 min for preliminary disinfection. In the ultra-clean workbench, use instruments such as scissors and tweezers after cleaning and sterilization to conduct experiments. The leg bones of the mouse limbs were taken out under aseptic conditions, soaked in PBS containing 10% penicillin & streptomycin (double antibody), and the muscles and connective tissue on the surface of the leg bones were removed as much as possible with a scalpel. The treated leg bones were placed in incomplete medium RPMI1640, the joints at both ends of the leg bones were cut off, and bone marrow cells (Bone Marrow Cell, BMC) were exposed. Using the prepared 1 m...
Embodiment 2
[0054] The anti-tumor effect of Exo-OVA-aPD-L1 was studied, and the results are as follows:
[0055] (1) Evaluation of the immune activation ability of Exo-OVA-aPD-L1 in vitro
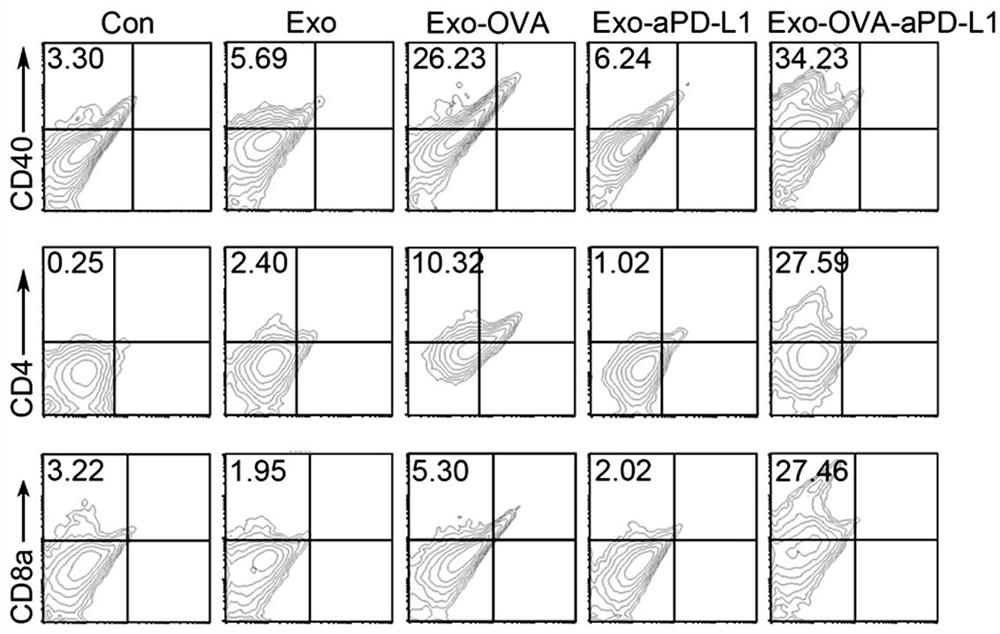
[0056] By isolating mouse spleen lymphocytes and detecting the number of proliferation and differentiation of lymphocytes, the ability of the material to activate immunity in vitro was verified. After incubation of mouse splenic lymphocytes with Exo, Exo-OVA, Exo-OVA-aPD-L1, the cells were resuspended in PBS by centrifugation, and T cells, DCs, B cells and macrophages were labeled with fluorescent antibodies respectively. As detected by flow cytometry, the results were as follows Figure 6 As shown, the first column in Figure a is CD8+ T cells, and the second column is CD4+ T cells. Figure b shows dendritic cells, picture c shows macrophages, and picture d shows B cells. The results showed that Exo was basically the same as the control group, without the ability to activate the immune system. Both ...
Embodiment 3
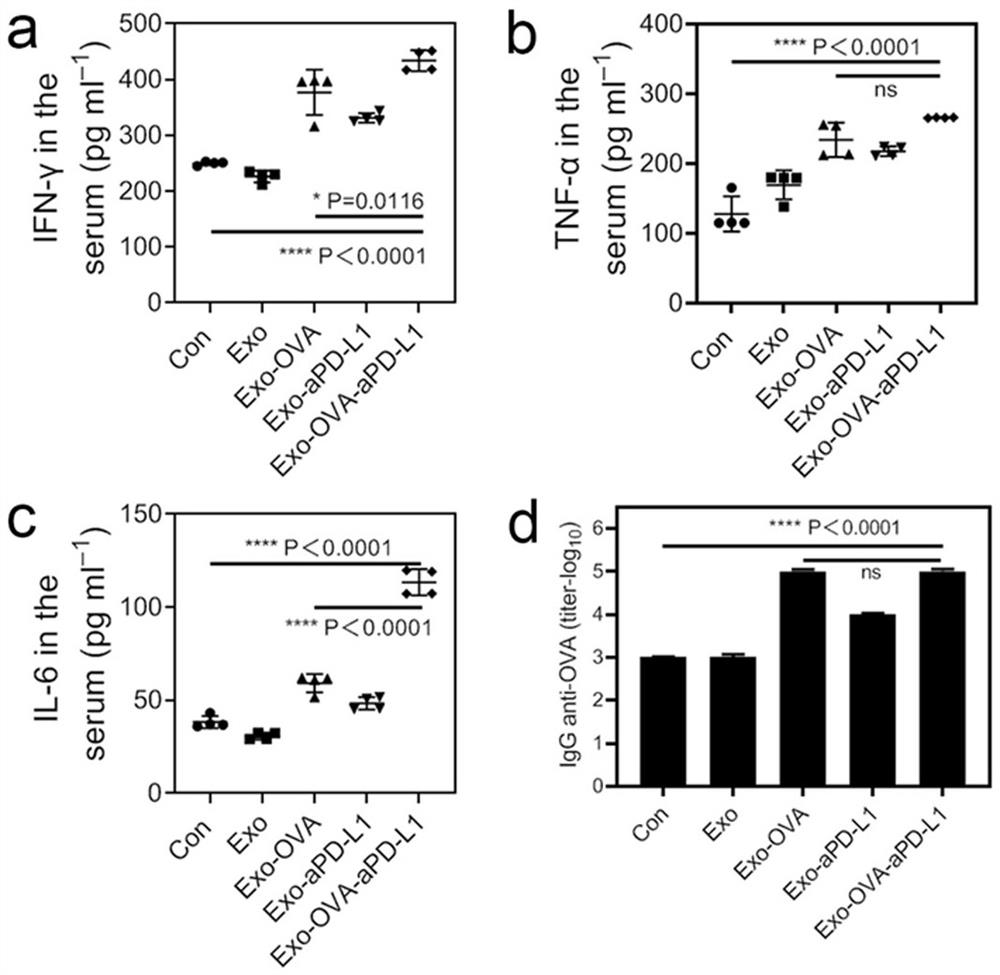
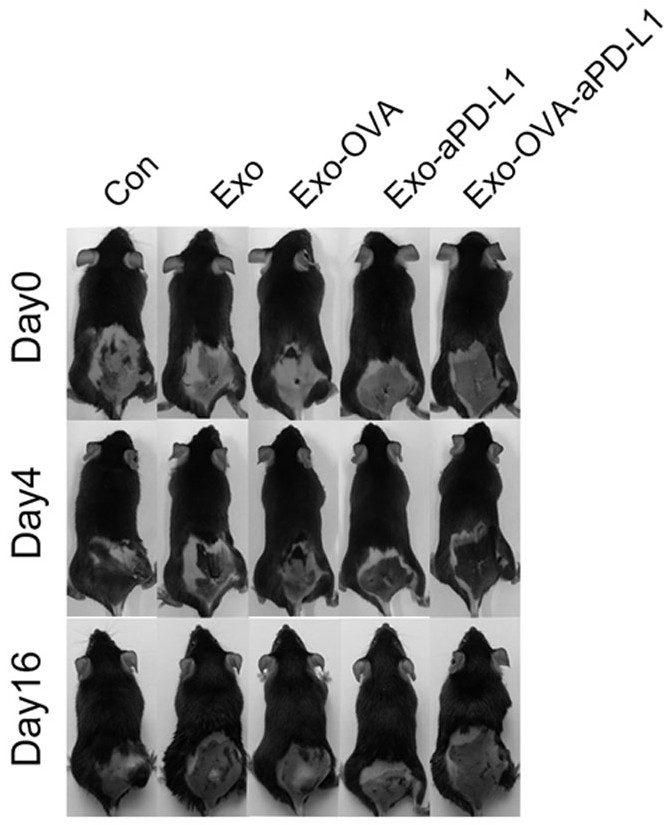
[0067] The anti-tumor effect of cross-linked Exo-OVA-aPD-L1 combined with pure exosome Exo-OVA and immune checkpoint drug aPD-L1 was studied. The results are as follows: Figure 14 and Figure 15 shown.
[0068] Through the anti-tumor effect in mice and local tumor immunohistochemical analysis, it can be proved that the cross-linked material is more effective in activating immunity, enhancing local immune cell infiltration in tumor, and effectively inhibiting tumor growth than simple drug combination therapy.
[0069] 1. DC cells are antigen-presenting cells, which express PD-L1 themselves, and exosomes secreted by them also express PD-L1. During antigen presentation, they form an immunosuppressive pathway with PD-1 on the surface of T cells, which will affect DC and Efficiency of exosomes in activating immune cells. By modifying the PD-L1 antibody, we can block its own PD-L1, and at the same time modify the surface of the PD-L1 antibody to enhance antigen presentation and i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com