Therapeutic medication for cartilage disorder
A cartilage and disease technology, applied in the field of pharmaceutical compositions for the prevention and/or treatment of cartilage diseases, can solve problems such as inability to cope with large defects, inability to naturally regenerate, and failure to establish a root therapy for traumatic articular cartilage defects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
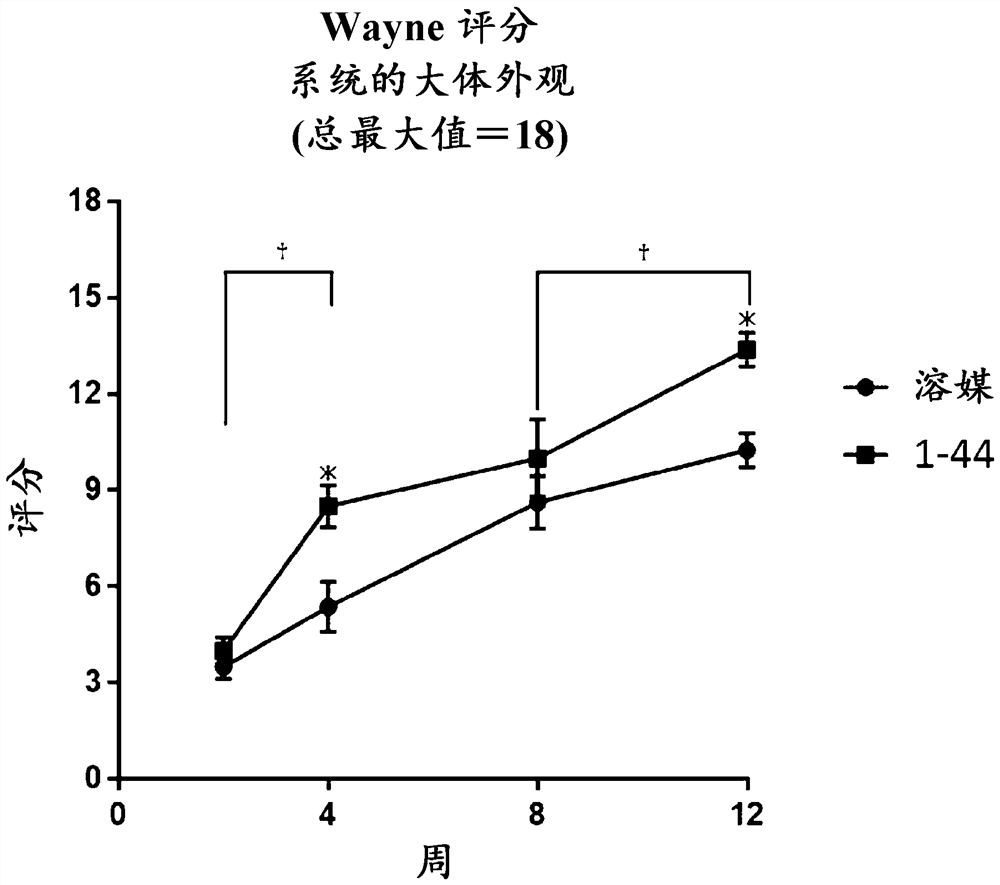
[0072] Evaluation of the Efficacy of HMGB1 Fragment Peptides on Articular Cartilage Defects
[0073] (1) Materials and methods
[0074] i) Preparation of medicine
[0075] A peptide consisting of amino acid residues 1-44 (SEQ ID NO: 1) of human HMGB1 protein was chemically synthesized by solid phase method. Hereinafter, the HMGB1 fragment peptide is referred to as HMGB1 peptide (1-44), and in the figures corresponding to the examples, the peptide is simply indicated as "1-44".
[0076] ii) Generation of cartilage defect model mice
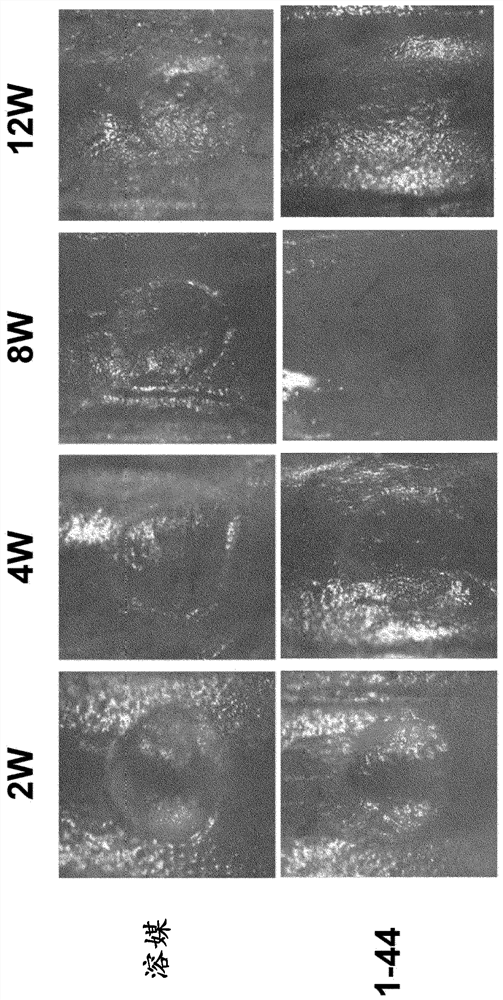
[0077] C57BL / 6J mice (6-7 weeks old, male, wild type) were purchased from CLEA Japan, Inc., and then adapted to the animal facility until reaching 8 weeks of age (about 25 g at 8 weeks of age). While mice were under 1.5-2.0% (v / v) isoflurane inhalation anesthesia, the lateral lower limbs were shaved to create cartilage defects, and a longitudinal skin incision of approximately 1 cm was applied with scissors in the middle of the knee joint . Th...
Embodiment 2
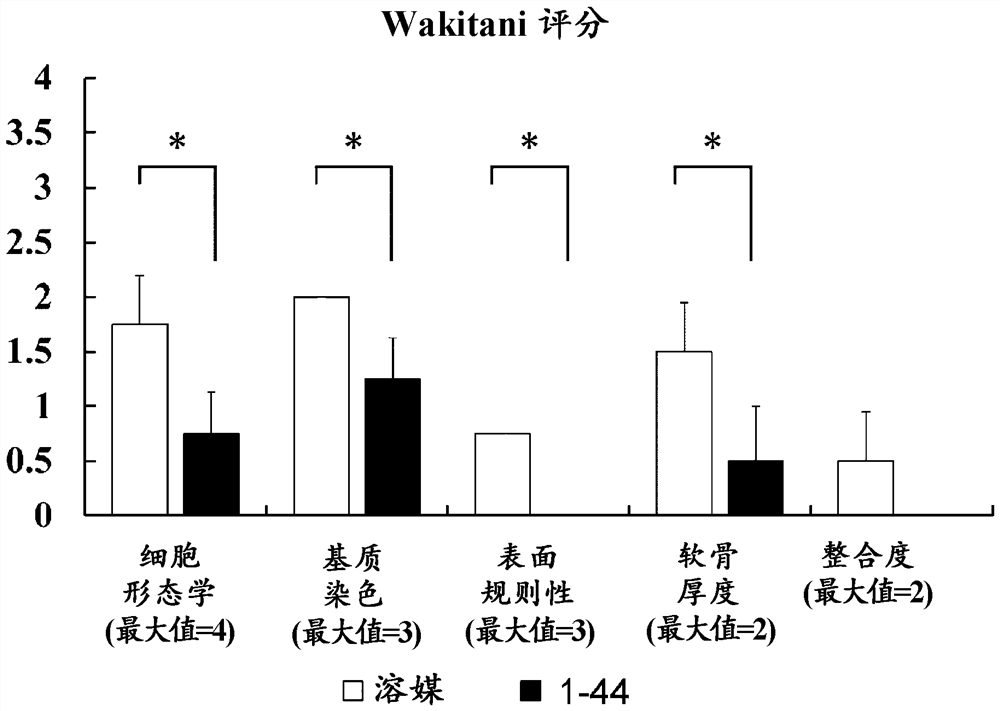
[0093] Histological staining of articular cartilage
[0094] method
[0095] Cartilage defect model mice were produced in the same manner as in Example 1 and continued to be bred. Tissue at the cartilage defect-generating site including the knee joint was removed from the mice at 12 weeks after the operation to produce paraffin sections, and the sections were subjected to immunostaining and safranin O staining with an anti-type II collagen antibody. In addition, cartilage tissues of knee joints were removed from normal mice of the same strain to produce paraffin sections, and type II collagen immunostaining and safranin O staining were performed on the sections in the same manner.
[0096] result
[0097] In the cartilage defect model mice, the tissue at the site of the cartilage defect in the knee joint at 12 weeks after operation (i.e., the tissue that was allowed to recover naturally after the cartilage defect was generated) was hardly shown in either safranin O staining ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com