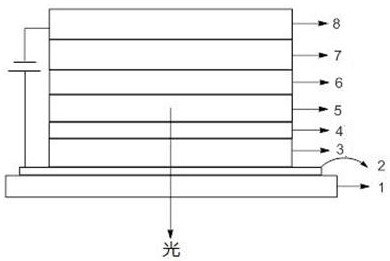
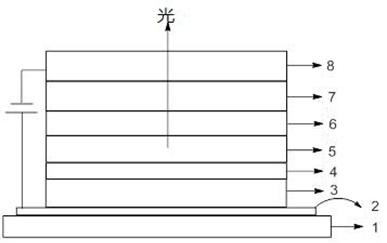
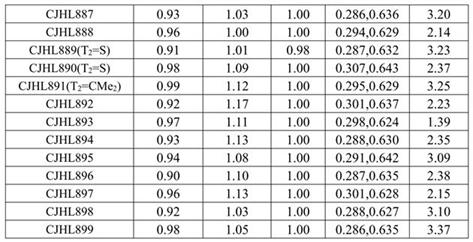
Thioxanthene derivatives and their applications
A derivative, thioxanthene technology, applied in the field of organic electroluminescent materials, can solve the problems of low thermal stability and high driving voltage, reduce the starting voltage, improve luminous efficiency and brightness, improve thermal stability and transport electrons. effect of ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The preparation method of compound CJHL814 comprises the following steps:
[0081] The first step: preparation of intermediate Int-1
[0082]
[0083] 50.0 mmol of 4-bromo-1-mercaptonaphthalene (CAS: 62648-52-6), 75.0 mmol of tolan (CAS: 501-65-5), 2.5 mmol of [(Cp*RhCl 2 ] 2 Catalyst (CAS: 12354-85-7), 0.1 mol of copper acetate monohydrate and 10.0 mmol of anhydrous sodium acetate were put into a pressure-resistant reactor, then 200 mL of dry acetonitrile was added, and the temperature was raised to 130°C under the protection of nitrogen The reaction was stirred for 12 hours, cooled to room temperature, concentrated to dryness under reduced pressure, separated and purified by silica gel column to obtain Int-1 as a yellow solid, yield: 46%.
[0084] The second step: the preparation of intermediate Int-2
[0085]
[0086] 50.0 mmol of Int-1 was dissolved in 120 mL of dichloromethane, under nitrogen protection, 5.0 mmol of anhydrous ferric chloride was added, sti...
Embodiment 2
[0105] The preparation of compound CJHL830 (with T 2 =CMe 2 example):
[0106]
[0107] 20.0 mmol of Int-2, 18.0 mmol of N-([1,1'-biphenyl]-4-yl)-9,9-dimethyl-9H-fluoren-2-amine (CAS: 897671-69- 1), 0.2 mmol of Pd 2 (dba) 3 Catalyst, 0.4 mmol of Xanphos and 30.0 mmol of sodium tert-butoxide, then add 80 mL of dry toluene, under the protection of nitrogen, raise the temperature to 100 °C and stir for 12 hours, then cool down to room temperature, add 50 mL of water, and use ethyl acetate Extract, collect the organic phase, concentrate to dryness under reduced pressure, separate and purify with silica gel column, and recrystallize with dichloromethane-ethanol to obtain yellow solid CJHL830, yield: 78%, MS (MALDI-TOF): m / z 694.2582[ M+H] + ; 1 HNMR (δ, CDCl 3 ): 8.92~8.90 (1H, d); 8.86~8.84 (1H, d); 8.55~8.53 (1H, d); 8.33~8.27 (2H, m); 7.93~7.91 (1H, d); 7.69~7.57 (6H, m); 7.53~7.48 (4H, m); 7.45~7.38 (5H, m); 7.36~7.28 (5H, m); 7.26 (1H, s); 7.21~7.17 (2H, m); 1.68 (...
Embodiment 3
[0114] Preparation of compound CJHL847:
[0115]
[0116] Mix 50 mL of DMSO and 30.0 mmol of potassium hydroxide, stir at room temperature for 30 minutes, add 18.0 mmol of 9H-3,9'-biscarbazole (CAS: 18628-07-4), stir for 30 minutes, and then Add 20.0 mmol of Int-4, under the protection of nitrogen, raise the temperature to 80°C and stir for 5 hours, cool down to room temperature, add 200 mL of water, filter, wash the filter cake with water and ethanol, separate and purify with silica gel column, and then use two Chloromethane-THF recrystallization gave yellow solid CJHL847, yield: 88%, MS (MALDI-TOF): m / z 666.2018[M+H] + ; 1 HNMR (δ, CDCl 3 ): 8.93~8.91 (1H, d); 8.86~8.84 (1H, d); 8.63~8.61 (1H, d); 8.36~8.34 (1H, d); 8.20 (1H, s); , m); 7.96 (1H, s); 7.89~7.79 (3H, m); 7.71~7.62 (2H, m); 7.58~7.44 (3H, m); 7.38~7.23 (9H, m); 7.21~7.17 (1H, m).
[0117] With reference to the above-mentioned similar synthetic method, the following compounds were prepared:
[0118]
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com