Application of leonurine in preparation of anti-hepatitis B virus drugs
A technology of leonurine and hepatitis B virus, applied in the field of medicine, can solve the problems of multiple side effects of interferon, high virus rebound rate, low HBsAg clearance rate and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
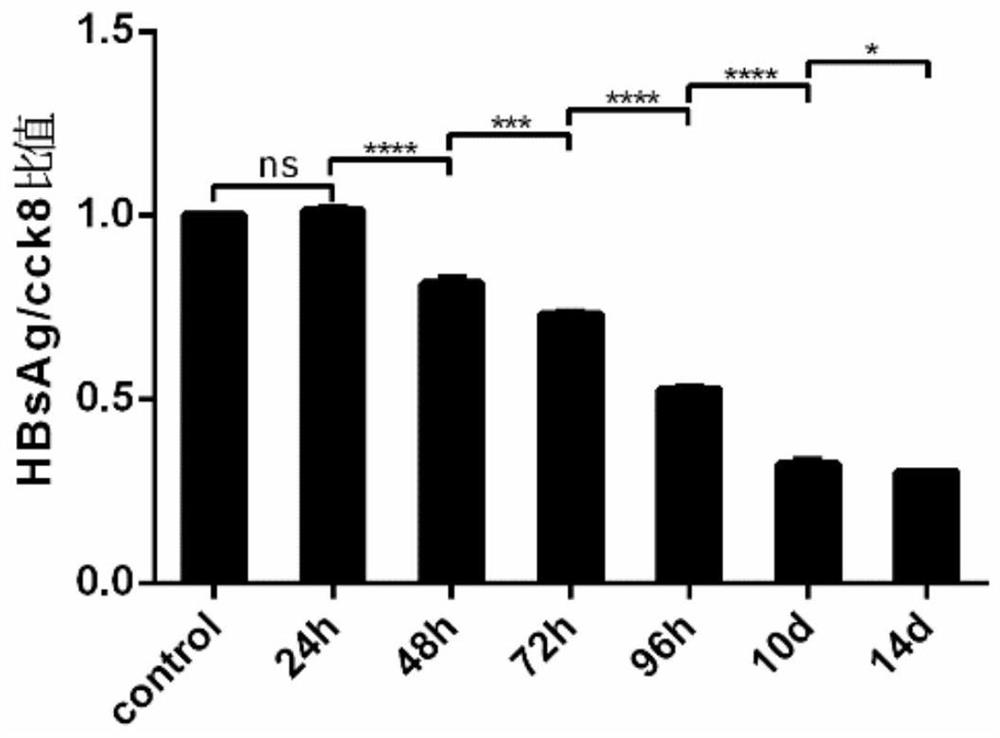
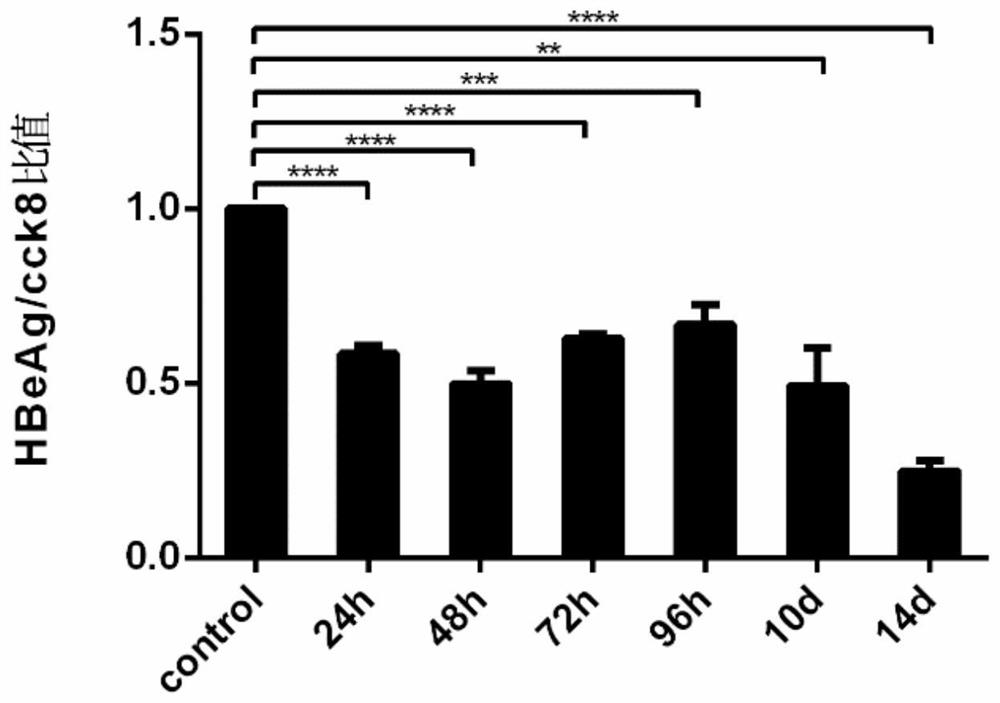
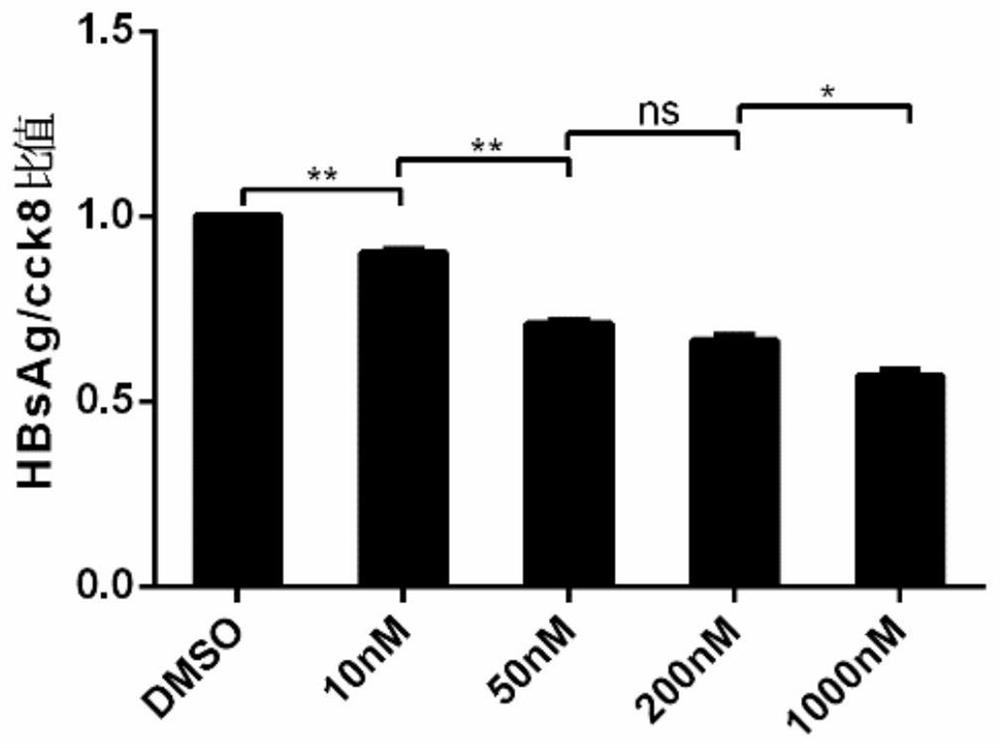
[0031] Hep G2.2.15 cell line: The human hepatoblastoma cell line Hep G2.2.15 stably expressing HBV was used. HepG2.2.15 cells were placed at 37°C, 5% concentration of CO 2 Cultured under conditions, screened and passaged under G418 drug pressure, all experiments were carried out in accordance with cell experiment specifications, and obtained permission from the Molecular Virus Laboratory of Fudan University School of Basic Medical Sciences, all cell grouping experiments were performed with the same starting conditions and random grouping conduct.
[0032] Drugs: G418 Sulfate (450-130-ZL, WISENT, 20mL) was used to maintain the continuous and stable expression of HBsAg and HBeAg in Hep G2.2.15 cells, and Leonurine (24697-74-3, HPLC>98%) and Lamivudine (134678-17 -4, Macklin, 5g) were dissolved in DMSO (D2650, Sigma-Aldrich, 100mL), and evenly added to the cell culture medium, and the different dose groups were adjusted to the corresponding final concentration gradient of mother...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com