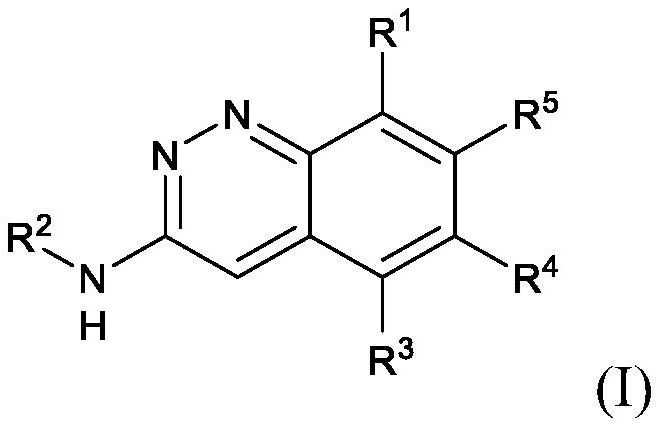
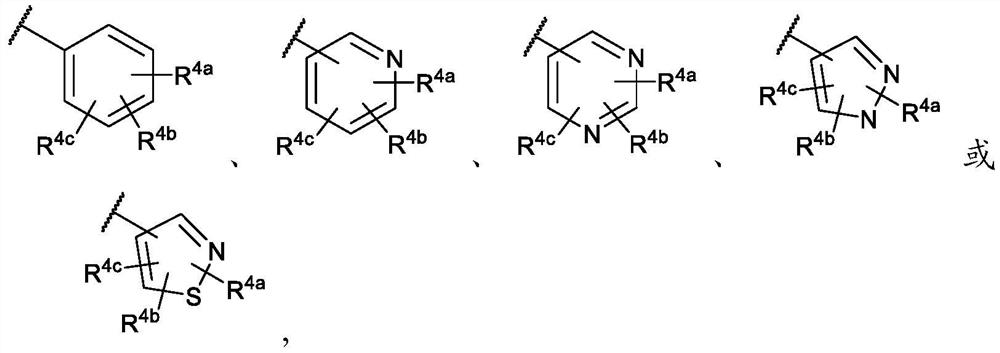
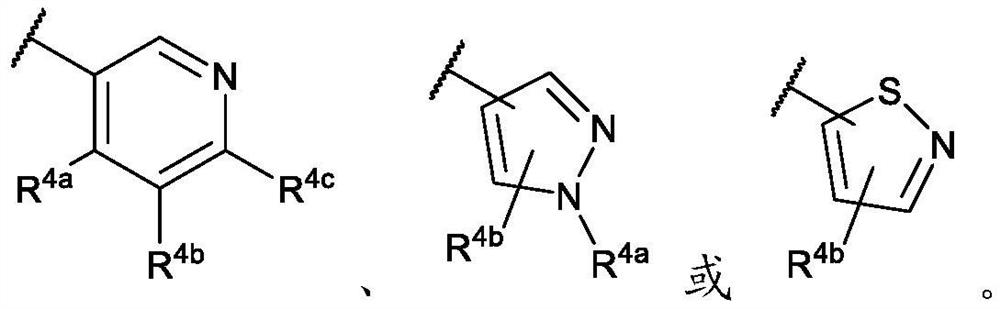
Cinnoline compounds and for the treatment of hpk1-dependent disorders such as cancer
A technology of compounds and medicinal salts, applied in the field of cinnoline compounds and HPK1-dependent diseases such as cancer, which can solve problems such as damage to healthy cells, drug resistance, and easy mutation of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0385] abbreviation
[0386]
[0387]
[0388] All samples were pre-purified by an achiral system and checked for purity prior to chiral purification by SFC.
[0389] General analysis method
[0390] LCMS method
[0391] Method A: Agilent 1100HPLC coupled with Agilent MSD mass spectrometer was used in the experiment. The system used ESI as the ion source, Agilent SunFire-C18 chromatographic column (3.5μm, 4.6×50mm), and the flow rate was 2.0mL / min. The solvent system was a gradient starting with 95% of 0.05% TFA in water (solvent A) and 5% of 0.05% TFA in acetonitrile (solvent B) to 100% solvent B in 1.3 minutes. The final solvent system was held constant for 1.2 minutes.
[0392] Method B: Agilent 1200HPLC coupled with Agilent MSD mass spectrometer was used in the experiment. The system used ESI as the ion source, Agilent SunFire-C18 chromatographic column (3.5μm, 4.6×50mm), and the flow rate was 2.0mL / min. The solvent system was a gradient starting with 95% 0.01% T...
example I-1
[0403] Intermediate 1: 6-Bromo-8-chlorocinnolin-3-amine
[0404]
[0405] Step 1: (4-Bromo-2-chlorophenyl)hydrazine
[0406]
[0407] To a mixture of 4-bromo-2-chloroaniline (5 g, 24 mmol) in concentrated hydrochloric acid (9 mL) was added NaNO dropwise at 0 °C 2 (1.8g, 26mmol) in water (8mL). The mixture was stirred at 0 °C for 1 h. Add SnCl to the reaction mixture 2 (9g, 48mmol) in concentrated hydrochloric acid (16mL). The mixture was stirred overnight at room temperature. The reaction mixture was then cooled to 0 °C and 40% NaOH solution was added to adjust the mixture to pH=8. The mixture was extracted with EtOAc (500 mL x 2). The organic layer was washed with brine (200 mL), washed with Na 2 SO 4 Dry, filter and concentrate. Diethyl ether (100 mL) and 5 drops of methanol were added. The resulting slurry was filtered to give the desired (4-bromo-2-chloro-phenyl)hydrazine (4.8 g, 49% yield) as a yellow solid. LCMS(ESI)[M+H] + = 221.1.
[0408] Step 2: N'...
example 1
[0415] (1S,2S)-N-(8-amino-6-(1-ethyl-1H-pyrazol-4-yl)cinnolin-3-yl)-2-fluorocyclopropanecarboxamide (compound 1a)
[0416]
[0417] Step 1: 8-Chloro-6-(1-ethyl-1H-pyrazol-4-yl)cinnolin-3-amine
[0418]
[0419] 1-Ethyl-1H-pyrazole-4-boronic acid (86mg, 0.39mmol), Pd(dppf)Cl 2 (28mg, 0.04mmol) and K 2 CO 3 (160 mg, 1.2 mmol) was added successively to a solution of 6-bromo-8-chloro-cinnolin-3-amine (200 mg, 0.39 mmol) in 1,4-dioxane (15 mL) and water (2 mL). The reaction mixture was stirred overnight at 100°C, then filtered. Partition the filtrate in H 2 O (10mL) and CH 2 Cl 2 (2×10mL). The combined organic layers were washed with Na 2 SO 4 Dry, filter and concentrate. The residue was purified by reverse phase (C-18) column chromatography (A: containing 10 mM NH 4 HCO 3 , B: ACN) to give 8-chloro-6-(1-ethylpyrazol-4-yl)cinnolin-3-amine (50 mg, 47% yield) as a yellow solid. LCMS(ESI)[M+H] + = 365.1.
[0420] Step 2: (1S,2S)-N-(8-Chloro-6-(1-ethyl-1H-pyrazol-4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com