Cathode active material precursor for secondary battery, cathode active material, preparation method therefor, and lithium secondary battery comprising same
A cathode active material, secondary battery technology, used in secondary batteries, battery electrodes, lithium batteries, etc., can solve the problems of low electrochemical performance and density, low ionic conductivity, and reduced strength, and achieve high capacity, high Effects of density, high rate capability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] After putting 4 L of distilled water into a co-precipitation reactor (capacity 20 L), and then adding 100 ml of 28% by weight ammonia solution to the reactor while maintaining the temperature at 50° C., at 300 ml / hr and 42 ml / hr, respectively, Concentration is the solution containing transition metal cation of 3.2mol / L and the aqueous ammonia solution of 28% by weight to the reactor continuously at the rate of adding, in the solution containing transition metal cation so that nickel: cobalt: the mol ratio of manganese is 2:2:6 mixed with NiSO 4 、CoSO 4 and MnSO 4 . Stirring was performed at an impeller speed of 400 rpm, and in order to maintain the pH, 40% by weight sodium hydroxide solution was added to maintain the pH at 9.5.
[0095] In this case, oxygen (O 2 ) while co-precipitating for 24 hours to form 0.4(Ni 0.5 co 0.5 (OH) 2 )·0.6(MnO 2 ) precursor particles. The precursor particles were separated, washed, and then dried in an oven at 130° C. to prepare ...
experiment example 1
[0100] [Experimental Example 1: Precursor Particle Confirmation]
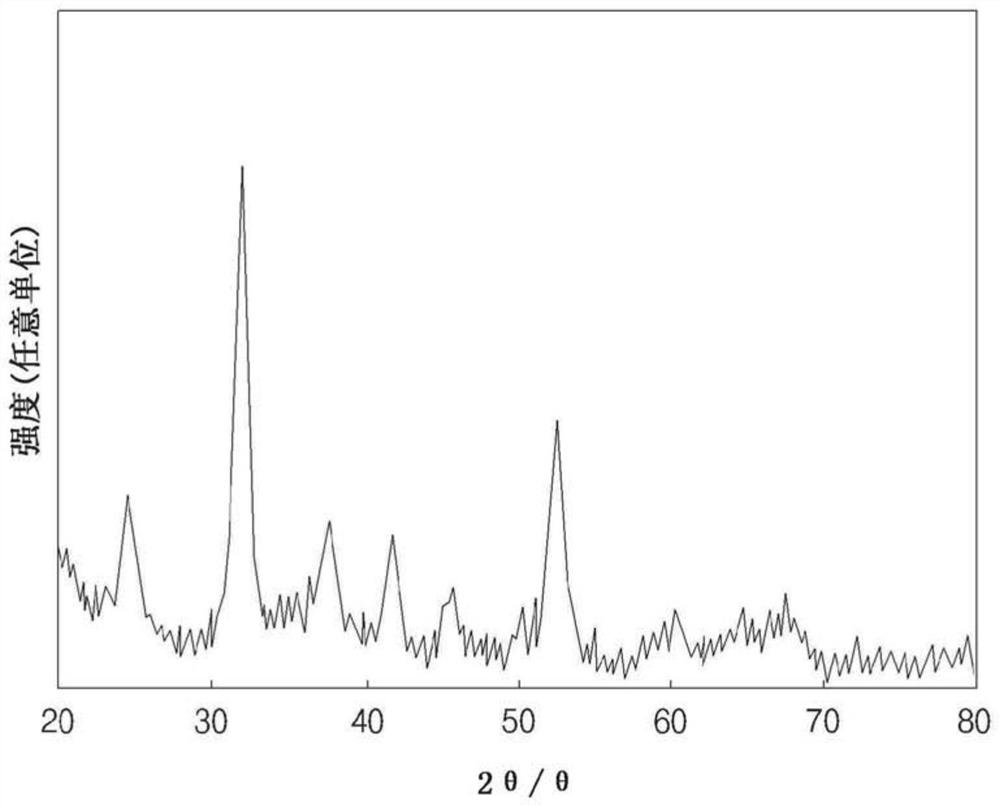
[0101] The cathode active material precursors prepared in Example 1 and Comparative Examples 1 and 2 were confirmed by using X-ray photoelectron spectroscopy (XPS) and X-ray diffraction (XRD). show the result in Figure 1 to Figure 4 middle. Specifically, in XPS analysis, by using ESCALAB 250 (Thermo Fisher Scientific) equipment obtained measurement scanning spectrum and narrow scanning spectrum, carried out XPS test, and in XRD analysis, using Cu target under the acceleration voltage of 40kV and the acceleration current of 40mA in the range of 10 ° ~ 90 ° per minute XRD measurements were performed at a rate of 3° using D4 ENDEAVOR (Bruker AXS GmbH) equipment.
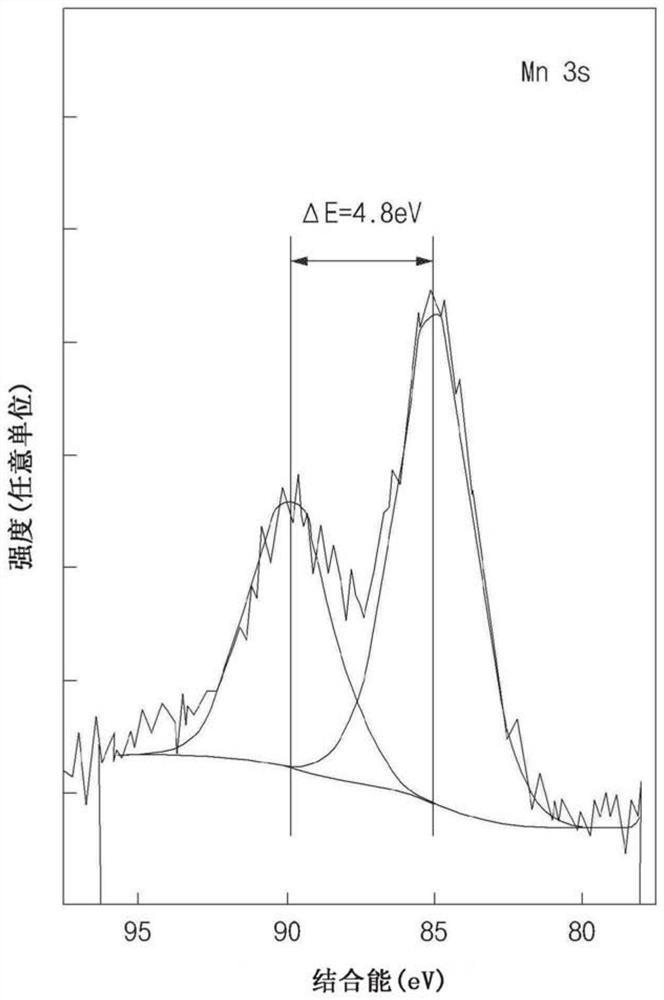
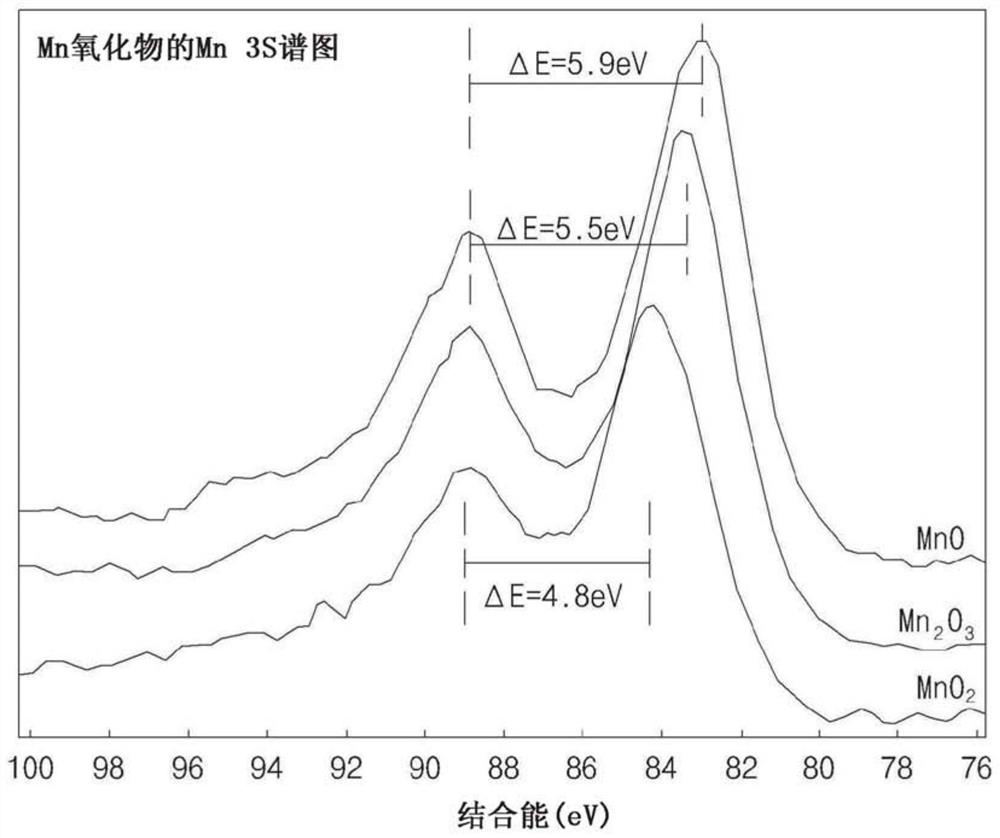
[0102] refer to figure 1 and figure 2 (XPS of embodiment 1( figure 1 ) and reference data ( figure 2 )), the oxidation number of Mn can be known from the energy difference of the two peaks separated in the XPS spectrum of Mn 3s, where, from ...
experiment example 2
[0105] [Experimental Example 2: Measurement of Tap Density]
[0106] By putting 50 g of various cathode active material precursors prepared in Example 1 and Comparative Examples 1 and 2 into a 50 ml graduated cylinder, and performing 1250 vibrations using a STAV-2 tap density meter (J. Engelsmann AG), the measured Tapped density. The results are shown in Table 1 below.
[0107] [Table 1]
[0108] Example 1 Comparative example 1 Comparative example 2 Tap density(g / cc) 1.55 1.24 1.37
[0109] Referring to Table 1, it can be confirmed that the tap density of the positive active material precursor of Example 1 is significantly higher than that of the positive active material precursors of Comparative Examples 1 and 2 in the form of hydroxide / carbonate, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com