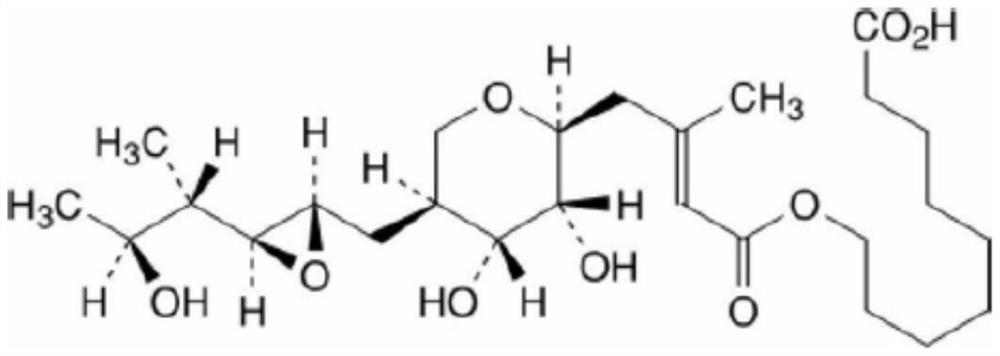
Mupirocin ointment preparation
A technology of mupirocin and preparations, applied in the field of pharmaceutical preparations, to achieve the effects of suitable consistency, good stability and good compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] composition Dosage (g) mupirocin 2 Sodium carboxymethyl cellulose 1 polyethylene glycol 400 58.6 polyethylene glycol 3350 38.4
[0027] Preparation:
[0028] (1) When heating 29.3g polyethylene glycol 400 to 55°C, add polyethylene glycol 3350 and stir, continue to heat up to 75°C until polyethylene glycol 400 and polyethylene glycol 3350 melt to obtain a mixed solution, set aside;
[0029] (2) Mix mupirocin and sodium carboxymethylcellulose uniformly to obtain a mixture, and at a temperature of 55° C., add the mixture to 29.3 g of polyethylene glycol 400 and mix uniformly to obtain a drug-containing liquid;
[0030] (3) At a temperature of 55°C, mix the mixed solution and the drug-containing liquid evenly, cool to 40°C, and fill.
Embodiment 2
[0032] composition Dosage (g) mupirocin 5 Sodium carboxymethyl cellulose 3 polyethylene glycol 400 50 polyethylene glycol 3350 45
[0033] Preparation:
[0034] (1) When heating 30 g of polyethylene glycol 400 to 50°C, add polyethylene glycol 3350 and stir, and continue to heat up to 70°C until polyethylene glycol 400 and polyethylene glycol 3350 are melted to obtain a mixed solution, which is set aside;
[0035] (2) Mix mupirocin and sodium carboxymethylcellulose uniformly to obtain a mixture, and at a temperature of 60° C., add the mixture to 20 g of polyethylene glycol 400 and mix uniformly to obtain a drug-containing liquid;
[0036] (3) At a temperature of 60°C, mix the mixed solution and the drug-containing liquid evenly, cool to 45°C, and fill.
Embodiment 3
[0038] composition Dosage (g) mupirocin 10 Sodium carboxymethyl cellulose 5 polyethylene glycol 400 70 polyethylene glycol 3350 15
[0039] Preparation:
[0040] (1) When 49g of polyethylene glycol 400 is heated to 53°C, add polyethylene glycol 3350 and stir, and continue to heat up to 72°C until polyethylene glycol 400 and polyethylene glycol 3350 are melted to obtain a mixed solution, which is set aside;
[0041] (2) Mix mupirocin and sodium carboxymethylcellulose uniformly to obtain a mixture, and at a temperature of 57° C., add the mixture to 21 g of polyethylene glycol 400 and mix uniformly to obtain a drug-containing liquid;
[0042] (3) At a temperature of 56°C, mix the mixed solution and the drug-containing liquid evenly, cool to 42°C, and fill.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com