A kind of monoclonal antibody fnab12 and its application
An antibody and carrier technology, applied in the field of biomedicine, can solve problems such as difficult to obtain protein molecules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
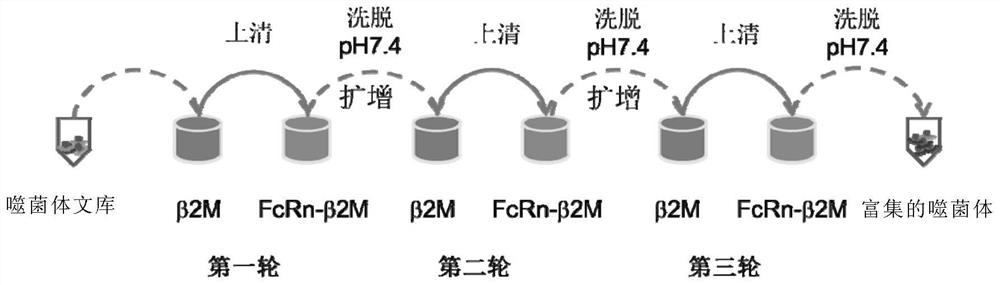
[0162] Example 1 Screening and Preparation of Monoclonal Antibodies
[0163] In this example, the constructed single-chain antibody phage library is screened, and the specific screening method is as follows: figure 1 As shown, that is, b2M and hFcRn&b2M are biotin-labeled first, then the biotin-labeled b2M is mixed with the phage antibody library for subtractive screening, and the phage bound to b2M is removed by magnetic beads coupled with avidin protein Finally, the supernatant was mixed with biotin-labeled hFcRn&b2M protein, and the phages bound to hFcRn were collected with magnetic beads. After amplification, the next round of panning was performed. After three consecutive rounds of panning, a single phage was picked and amplified. Apply phage enzyme-linked immunosorbent assay method to identify positive phages, then sequence them, clone the gene into a new expression vector, express and purify the antibody, and conduct subsequent identification of binding and affinity.
...
Embodiment 2
[0169] Preparation of embodiment 2 fusion protein
[0170] The nucleotide sequence expressing GLP1 (7-36 amino acids, containing A8G, G22E, and R36G three mutation sites) is connected to the 5' end of the nucleotide sequence of FnAb8 or FnAb12 with a (G4S)3 linker sequence, and the gene After the sequence was synthesized, it was inserted into the expression vector pcDNA3.1 (GenScript Biotechnology Co., Ltd.), and then the plasmid was transfected into mammalian cells to prepare the protein.
[0171] The amino acid sequence of the GLP1 (AA7-36) protein used in this example is as follows:
[0172] HGEGFTTSDVSSYLEEQAAKEFIAWLVKGG (SEQ ID NO.: 2)
[0173] In this example, GLP1 (AA7-36) was successfully fused and expressed with two strains of single-chain antibodies, and a fusion protein was prepared. The fusion protein produced by fusion expression with FnAb8 was named G8, and the fusion protein produced by fusion expression with FnAb12 Named G12.
[0174] The amino acid sequence...
Embodiment 3
[0178] In vitro activity detection of embodiment 3 fusion protein
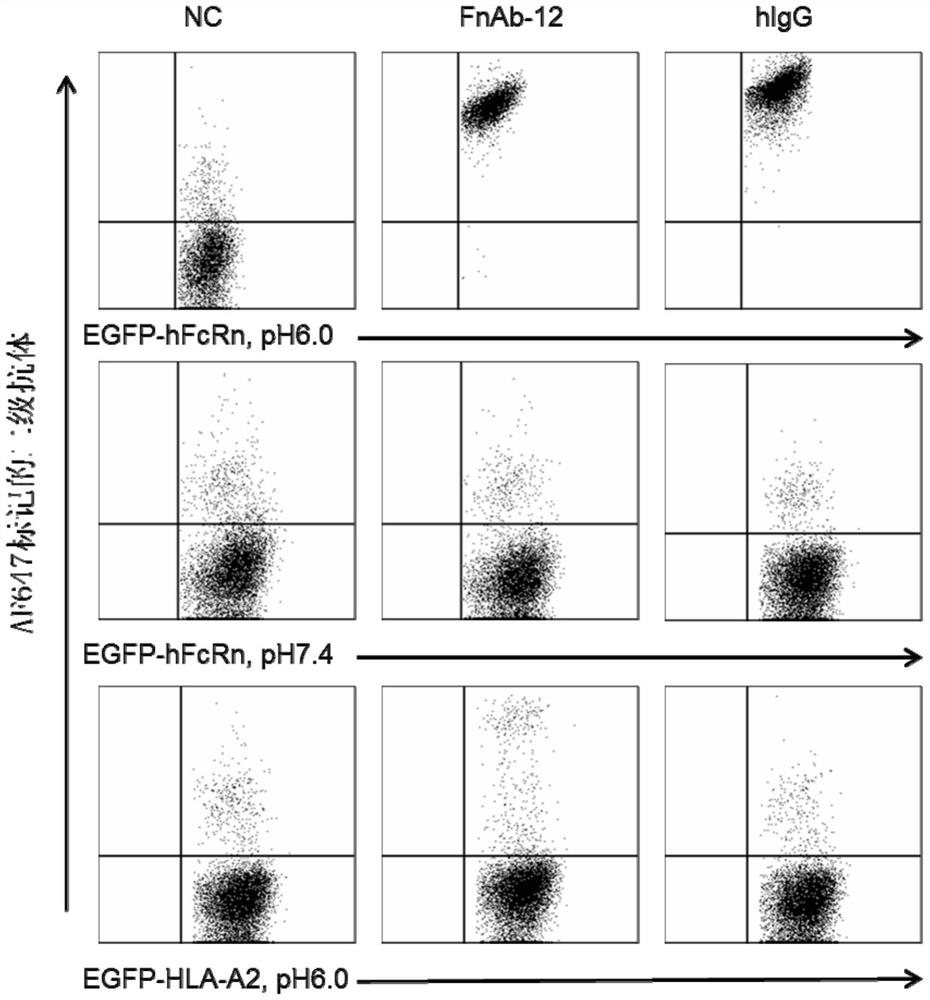
[0179] The combination of GLP-1 fusion protein and its corresponding receptor protein was identified by plasmon surface resonance (SPR). GLP-1R-Fc protein and hIgG were immobilized on the CM5 chip by chemical coupling using Biacore T100. 200nM FnAb8, G8, FnAb12 and G12 respectively flowed over the surface of the chip at high speed, and their binding signals were detected.
[0180] Biacore was used to identify the binding of GLP-1 fusion protein to hFcRn. hFcRn was coupled to a CM5 chip, and 200nM FnAb8, G8, FnAb12 and G12 were flowed over the surface of the chip at high speed to detect the binding signal.
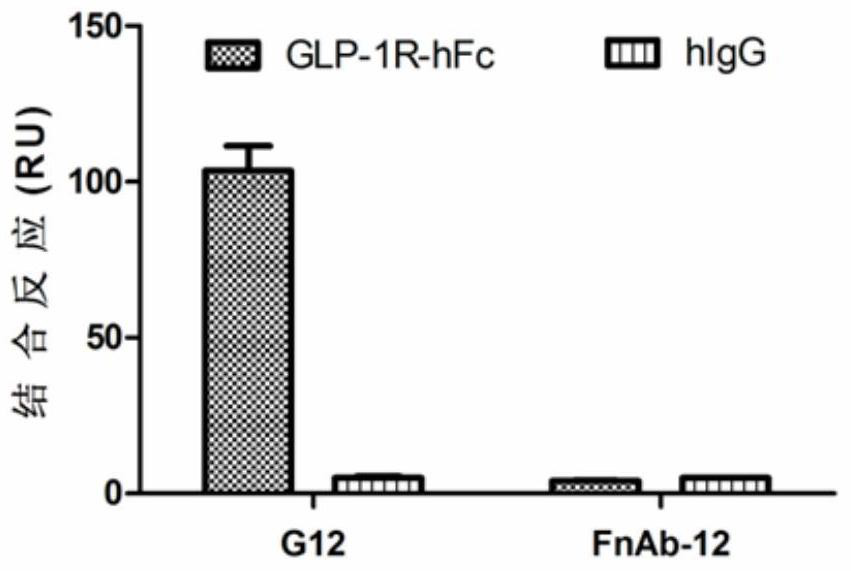
[0181] The experimental results in this example prove that G8 and G12 can specifically bind to GLP-1R. And both G8 and G12 can bind to FcRn protein, and maintain the pH dependence of the binding. image 3 Binding of G12 to GLP-1R-Fc protein is shown. GLP-1R-Fc or hIgG was coupled to the CM5 chip, and 200...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com