Chitosan microcapsule, its preparation method and uses
A technology of chitosan and chitosan solution, applied in the field of chitosan, can solve the problems of controlled release of unfavorable drugs, large particle size of chitosan microcapsules, loose microcapsule structure, etc., and achieve low cost and good particle size distribution Uniform, easy-to-process results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Chitosan was dissolved in 0.2mol / L acetic acid solution to prepare a 2% (w / v) solution, 50mL of liquid paraffin was added to the reactor, 1mL of Span 80 was added, and the stirring speed was 1500 rpm. After dispersing for 10 minutes, add 10 mL of chitosan solution and continue to stir and disperse for 20 minutes, then add 50 mL of acetone dissolved in 200 mg of vanillin for cross-linking reaction, and stop after 1 hour. The reaction mixture was centrifuged, the reaction product was fully washed with petroleum ether, and the microcapsules were obtained after vacuum drying at room temperature. It can be used to embed proteins, polypeptide active macromolecular drugs and common drugs for colon targeted drug delivery.
Embodiment 2
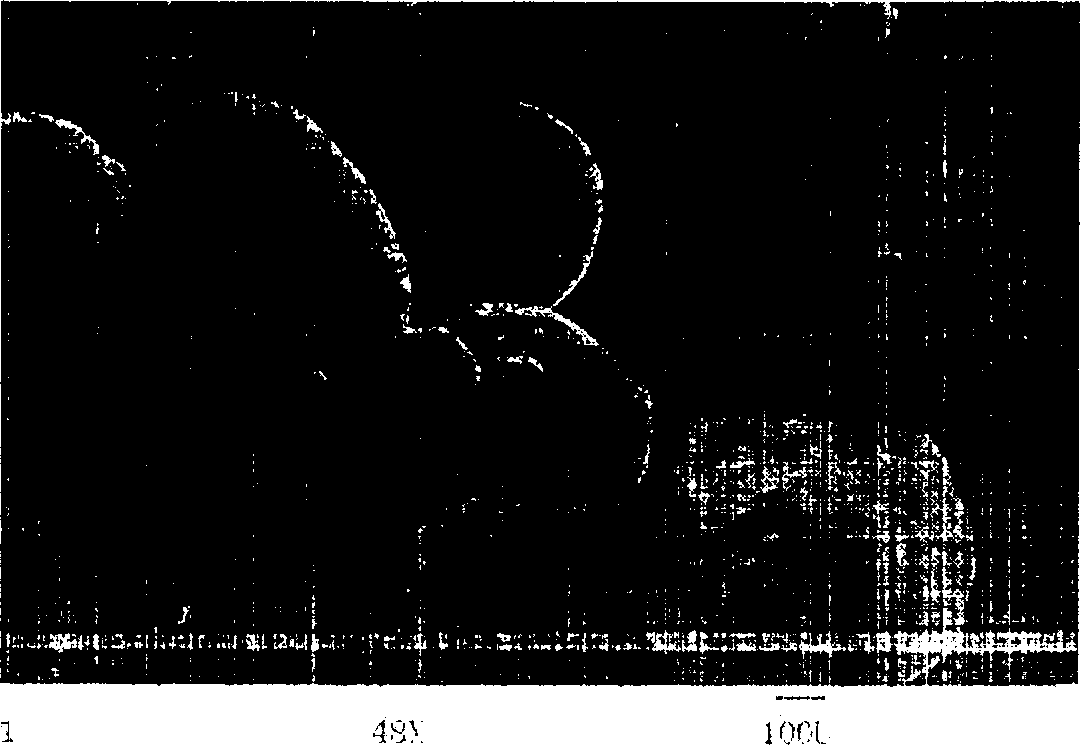
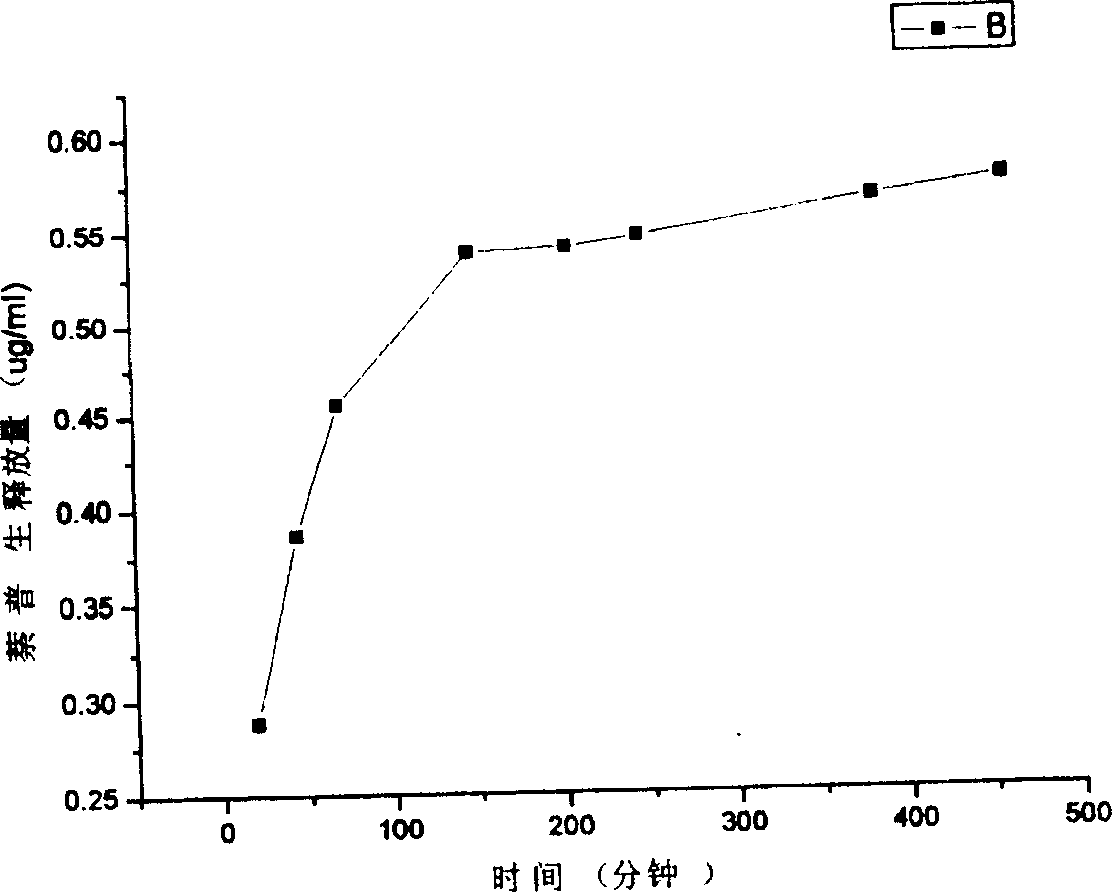
[0035] Chitosan was dissolved in 0.2mol / L acetic acid solution to prepare a 3% (w / v) solution, 100mg naproxen was dispersed in the above 10mL chitosan solution, stirred, and 50mL liquid paraffin was added to the reactor In the process, continue stirring at a speed of 1000 rpm. After 20 minutes, add 60 mL of acetone dissolved in 1 g of vanillin for cross-linking, and stop the reaction after 1.5 hours. The reaction mixture is centrifuged, and the reaction product is fully washed with sherwood oil, and after vacuum drying at room temperature, the chitosan microcapsules embedded with naproxen are obtained, and the microcapsule particle diameter is about 750um (such asfigure 1 ). The release profile of the microcapsules in a pH=5 buffer solution simulating the colonic environment is as follows: image 3 As shown, the microcapsule has the effect of colon-targeted drug release control.
Embodiment 3
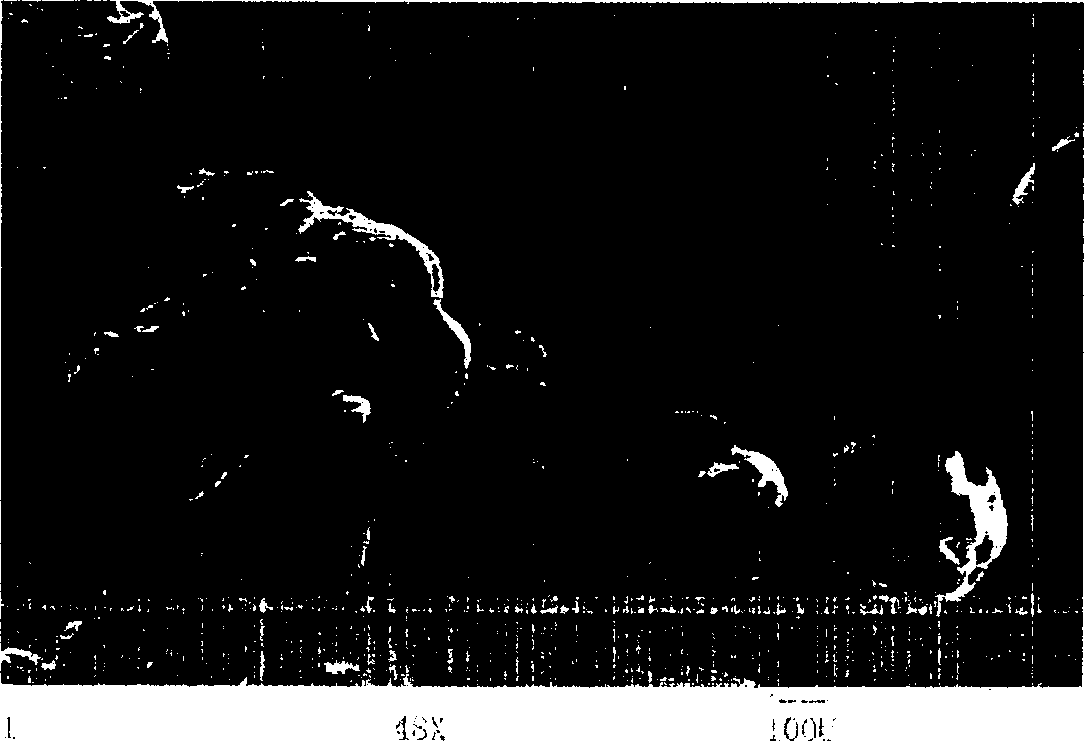
[0037] Chitosan was dissolved in 0.2mol / L acetic acid solution to prepare a 3% (w / v) solution, 100mg naproxen was dispersed in the above 10mL chitosan solution, stirred, and 50mL liquid paraffin was added to the reactor Add 1mL of Span 80, add the above dispersion system, continue stirring at 1000 rpm, after 20 minutes, add 60mL of acetone dissolved in 1g of vanillin for cross-linking, and stop the reaction after 1.5 hours. The reaction mixture is centrifuged, and the reaction product is fully washed with sherwood oil, and after vacuum drying at room temperature, the chitosan microcapsules embedded with naproxen are obtained, and the microcapsule particle diameter is about 430um (such as figure 2 ). The release profile of the microcapsules in a pH=5 buffer solution simulating the colonic environment is as follows: Figure 4 It shows that the microcapsule has the effect of colon-targeted drug release control.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com