Ph-responsive biodegradable polymer vesicles and its preparation method and application
A technology for degrading polymers and polymers, used in the fields of polymer chemistry and pharmaceutical biomedical engineering
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
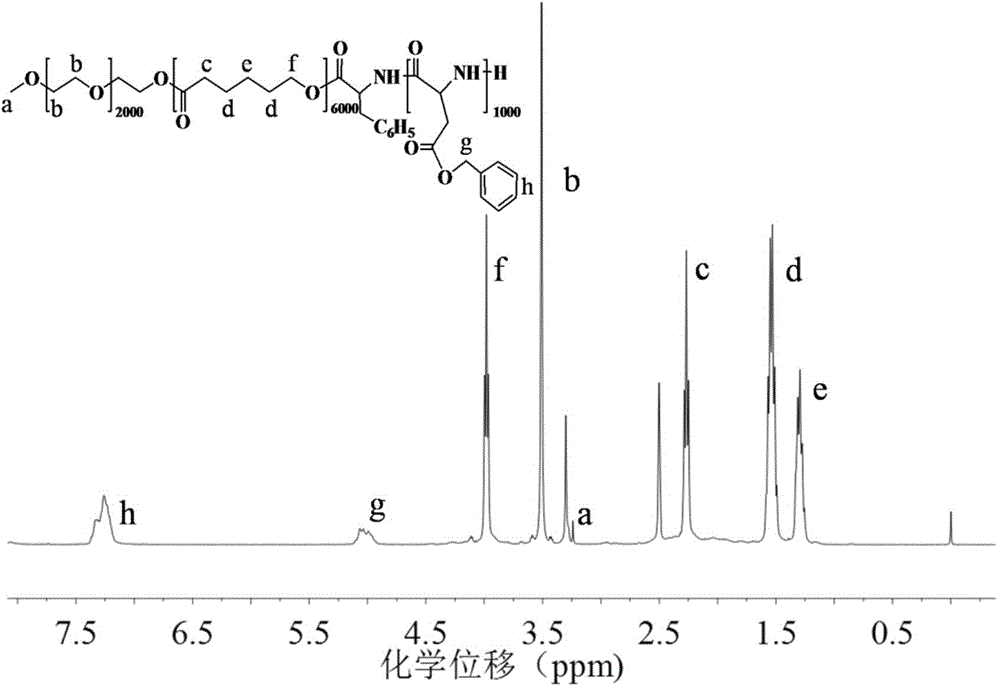
[0052] Example 1: Synthesis of pH-responsive triblock polymers
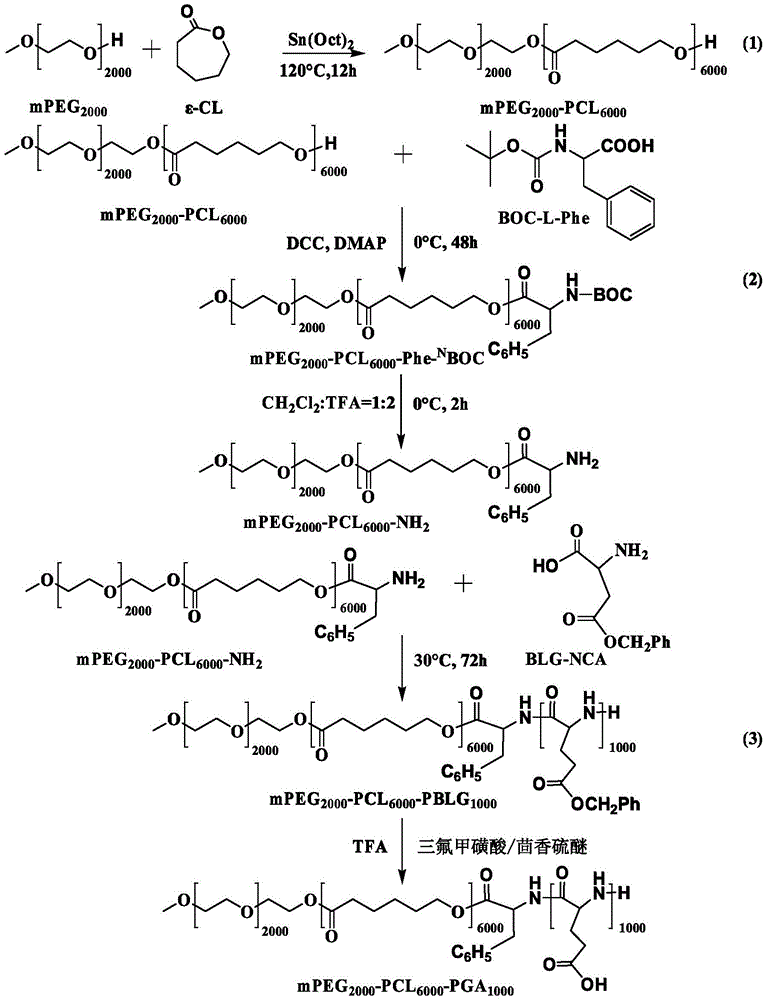
[0053] Synthetic route such as figure 1 As shown, the specific steps are as follows:
[0054] (1) Diblock copolymer mPEG 2000 -PCL 6000 Synthesis
[0055] Dissolve 20g of mPEG in 200ml of toluene, heat to boiling at 120°C, reflux for 10 hours, evaporate toluene, add 200ml of cold anhydrous ether to precipitate mPEG, filter, and store the dried mPEG in a vacuum oven.
[0056] 10gε-CL and 0.1gCaH 2 Put it in a 100ml round bottom flask, under magnetic stirring, reflux at 60°C to remove water for 12h, then distill ε-CL out under reduced pressure at 115°C; discard the remaining liquid in the flask.
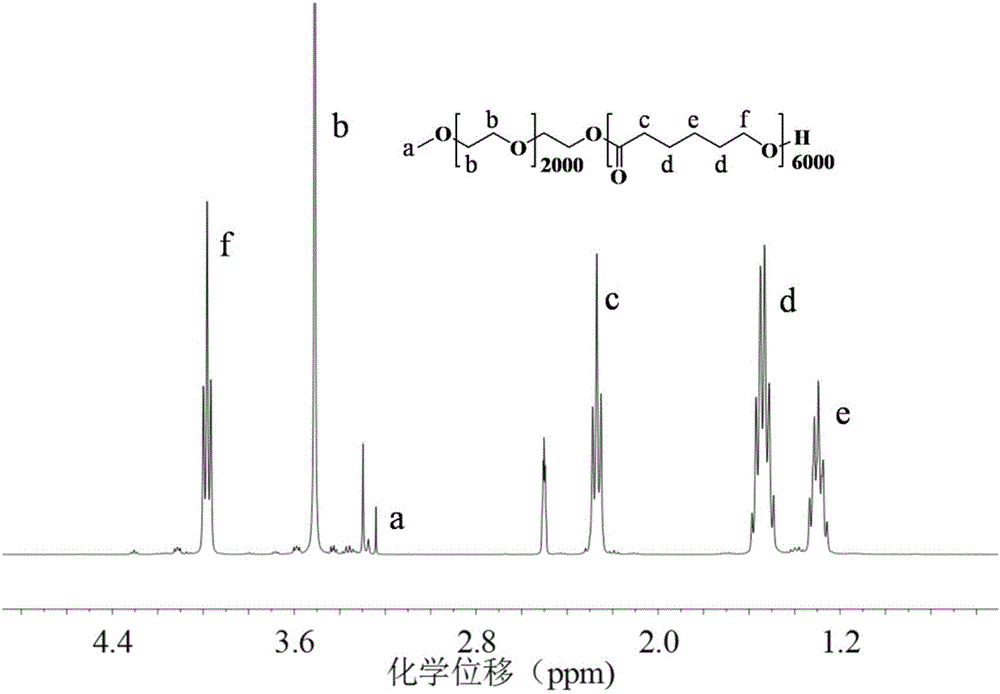
[0057] 2gmPEG (molecular weight: 2000Da), 6gε-CL and 24μl catalyst stannous octoate were added to the reaction flask, nitrogen (N 2 ) replacement 3 times, reacted at 120°C for 12h, cooled to room temperature, added 100ml of cold diethyl ether to precipitate the product, filtered and dried under vacuum to obtain mPE...
Embodiment 2
[0068] Example 2: Study on Aggregation Behavior of pH Responsive Triblock Polymers
[0069] The prepared mass concentration is 0.1-6mg·ml -1 A series of polymer (polymer is prepared in Example 1) solution, using a surface tension meter, 25 ℃, the surface tension of the sample is measured by the hanging method, the results are as follows Figure 5 As shown, the results show that this polymer can make the surface tension of water from 70mN·m -1 reduced to 45mN·m -1 , and its critical aggregation concentration is about 4 mg·ml -1 .
Embodiment 3
[0070] Example 3: Preparation of pH-responsive triblock polymersomes by nanoprecipitation method
[0071] 20mg of polymer (prepared in Example 1) was dissolved in 0.2mlDMSO (heatable to dissolve), then under stirring, phosphate buffer solution of 1.8mlpH7.4 was added dropwise thereto, and the mixed solution was transferred to a dialysis bag (MWCO 3500 Da ), and finally dialyzed in 500ml phosphate buffer solution (pH7.4) to remove DMSO to obtain pH-responsive triblock polymersomes. Dialysis was carried out for 4 hours, the medium was changed every 0.5 hours for the first 2 hours, and every 1 hour for the next 2 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com