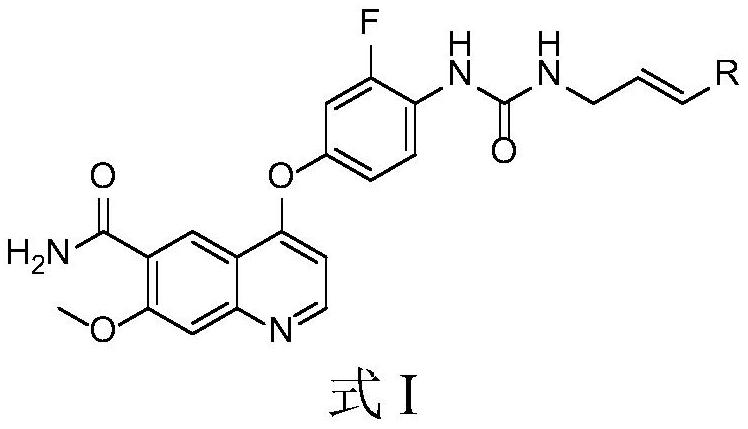
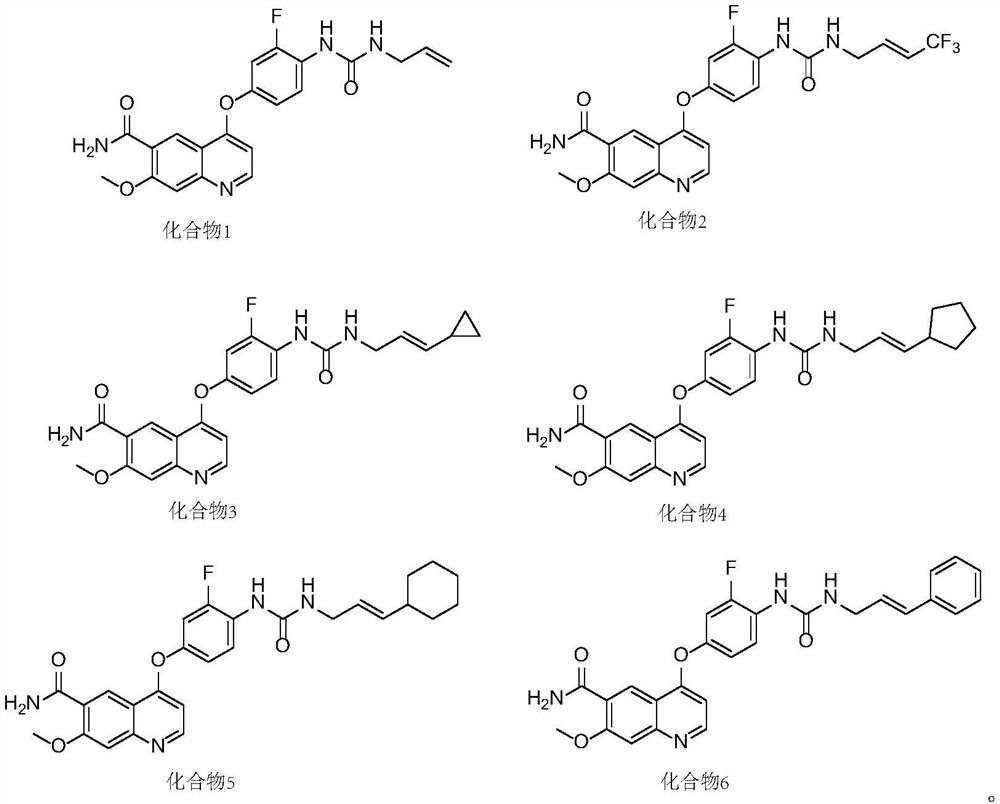
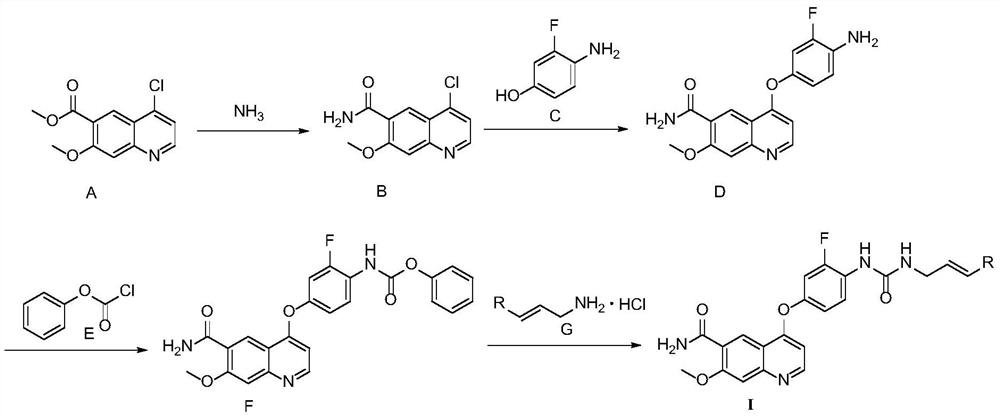
Multi-target tyrosine kinase inhibitor as well as preparation method and application thereof
A technology of tyrosine kinase and application, which is applied in organic chemistry, drug combination, antineoplastic drugs, etc., and can solve the problems of low efficacy and adverse reactions of lenvatinib
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1, the preparation of compound 1
[0043] The synthetic route is:
[0044]
[0045] The specific preparation methods include:
[0046] Add 12.6g (50.0mmol) of compound A (50.0mmol), ammonia-methanol solution (150.0mmol) and 50mL methanol to the reaction flask, react at 20-30°C for 16h, add 250mL water and 250mL ethyl acetate to the system, separate and collect the organic layer, organic The phase was washed successively with 100 mL of water, 50 mL of saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain Compound B. Purified by silica gel column (PE:EA=2:1) to obtain 7.9g, yield 67.0%, LC / MS m / z=237.2[M+H] + .
[0047] Dissolve 5.72g (45.0mmol) of compound C in 45mL DMF, slowly add 1.80g (45.0mmol) of hydrogen extraction agent NaH, stir at room temperature for 30min, then add 7.1g (30.0mmol) of compound B, react at 60°C for 4h, cool down to Add 250mL of water and 250mL of ethyl acetate at room temperat...
Embodiment 2
[0050] Embodiment 2, the preparation of compound 2
[0051] The synthetic route is:
[0052]
[0053] Synthesize F with the method of Example 1, take 0.70g (1.5mmol) of compound F, 0.48g (3.0mmol) of compound G-2, 0.45g (4.5mmol) of triethylamine and 5mL of DMF, and react the reaction system at 80°C After 4 hours, the temperature was lowered to room temperature, 25 mL of water and 50 mL of EA were added, and the organic phase was collected. The organic phase was washed with 10 mL of water and then concentrated to dryness under reduced pressure to obtain the crude compound 2, which was then purified by a silica gel column to obtain 0.27 g of the pure compound 2, with a yield of 38.0%. . LC / MS m / z=479.1[M+H] + , 1 HNMR(DMSO-d6):3.99(s,3H),4.26-4.28(m,2H),5.72-5.74(m,1H),6.12-6.14(m,1H),6.49(d,J=5.5Hz, 1H),7.21-7.24(m,2H),7.50-7.5(m,2H),7.85(s,1H),7.98(s,1H),8.25-8.26(m,1H),8.62-8.65(m, 2H).
Embodiment 3
[0054] Embodiment 3, the preparation of compound 3
[0055] The synthetic route is:
[0056]
[0057] Synthesize F with the method of Example 1, take 0.70g (1.5mmol) of compound F, 0.40g (3.0mmol) of compound G-3, 0.45g (4.5mmol) of triethylamine and 5mL of DMF, and react the reaction system at 80°C After 4 hours, the temperature was lowered to room temperature, 25 mL of water and 50 mL of EA were added, and the organic phase was collected. The organic phase was washed with 10 mL of water and concentrated to dryness under reduced pressure to obtain a crude compound 3, which was then purified by a silica gel column to obtain 0.28 g of a pure compound 3 with a yield of 41.0%. . LC / MS m / z=451.1[M+H] + , 1 HNMR(DMSO-d6):0.45-0.47(m,4H),1.30-1.32(m,1H),3.99(s,3H),4.26-4.28(m,2H),5.71-5.72(m,1H), 5.90-5.91(m,1H),6.48-6.49(m,1H),7.20-7.25(m,2H),7.49-7.52(m,1H),7.85(s,1H),7.99(s,1H), 8.25-8.27(m,1H),8.62-8.65(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com