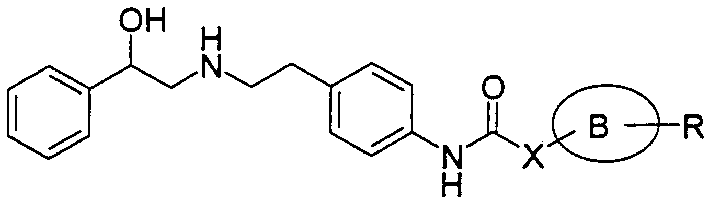
Imide derivatives ar Salts thereof
A technology of amide derivatives and salts, which is applied in the field of amide derivatives or their salts and therapeutic agents for treating diabetes, and can solve problems such as not indicating insulin secretion and enhancing insulin sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Add 4N hydrogen chloride-ethyl acetate solution (10ml) to 458mg of (R)-N-(2-hydroxy-2-phenylethyl)-N-[2-[4-[(2-pyridinecarbonyl)amino]benzene base] ethyl] tert-butyl carbamate in 10 ml ethanol solution. The reaction solution was stirred at room temperature for 3 hours, then the solvent was evaporated under vacuum. The obtained crude crystals were recrystallized from methanol-ethanol-ethyl acetate to obtain 289 mg of (R)-4'-[2-[(2-hydroxy-2-phenylethyl)amino]ethyl]-2-pyridine Carboxanilide Hydrogen Chloride.
Embodiment 2
[0083] (R)-2-[1-(4-chlorobenzyl)-1H-imidazol-2-yl]-4'-[2-[(2-hydroxyl-2-phenylethyl)amino]ethyl] Acetanilide Hydrogen Chloride
Embodiment 3
[0085] (R)-2-[1-(3,4-dichlorobenzyl)-1H-tetrazol-5-yl]-4'-[2-[(2-hydroxyl-2-phenylethyl)amino ]ethyl]acetanilide hydrochloride
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com