Preparation method of (5-fluoro-2, 3-dihydrobenzofuran-4-yl) methylamine or salt thereof and intermediate of (5-fluoro-2, 3-dihydrobenzofuran-4-yl) methylamine or salt thereof
A technology of fluorobenzofuran and dihydrobenzene, which is applied in the field of the preparation method of methylamine or its salt and its intermediates, can solve the problems of unsuitability for industrial production, high toxic price of raw material reagents, lengthy reaction route, etc., and achieve The post-processing is simple, environment-friendly, and the environment is cheap and easy to obtain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0085] In one aspect, the present invention provides a method for preparing (5-fluoro-2,3-dihydrobenzofuran-4-yl)methanamine (8) or a salt thereof, which comprises the following reaction steps:
[0086] 1) Using 4-fluoro-3-methylphenol (1) as an initial raw material, brominating it to obtain 2-bromo-4-fluoro-5-methylphenol (2);
[0087] 2) O-alkylation of 2-bromo-4-fluoro-5-methylphenol (2) with 2-bromo-1,1-diethoxyethane (3) to give 1-bromo- 2-(2,2-diethoxyethoxy)-5-fluoro-4-toluene (4);
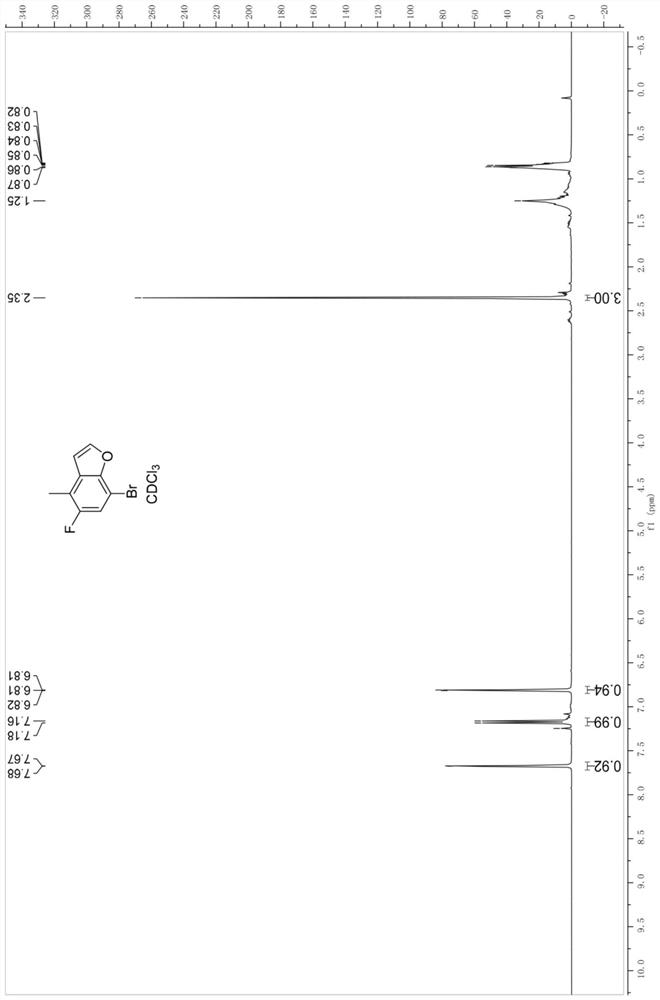
[0088] 3) Cyclization of 1-bromo-2-(2,2-diethoxyethoxy)-5-fluoro-4-toluene (4) to give 7-bromo-5-fluoro-4-methylbenzene And furan (5);
[0089] 4) bromination of 7-bromo-5-fluoro-4-methylbenzofuran (5) to give 7-bromo-4-(bromomethyl)-5-fluorobenzofuran (6);
[0090] 5) Prepare (5-fluoro-2,3-dihydrobenzofuran-4-yl)methanamine (8) or its salt by using any of the following two-step reactions in 5.1 or 5.2:
[0091] 5.1) Azidation of 7-bromo-4-(bromomethyl)-5-fluorobenzofuran (6) in the pre...
Embodiment 1
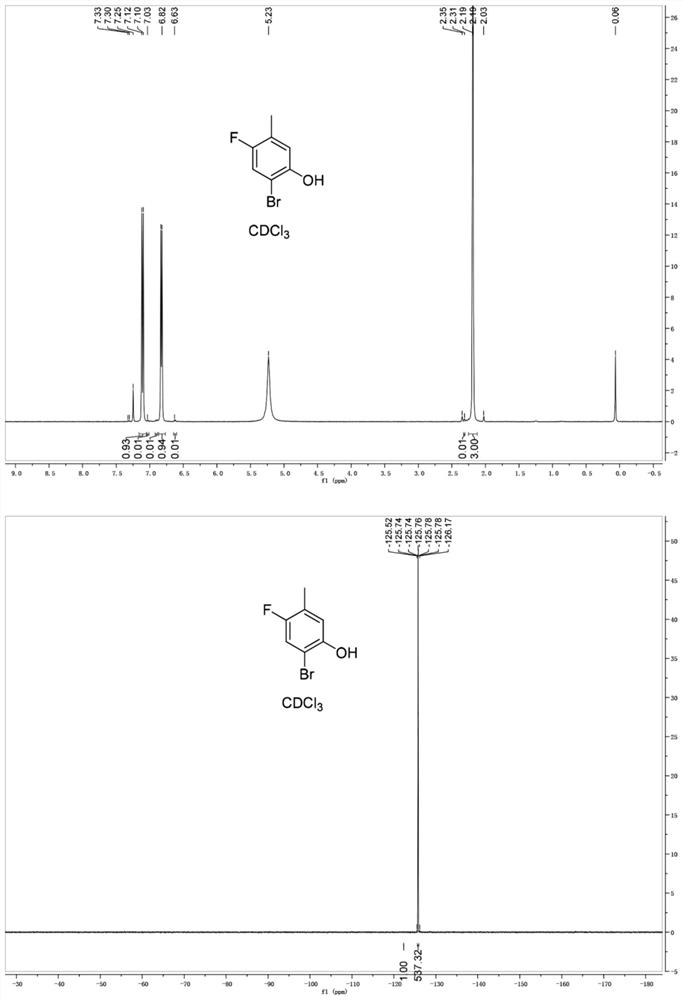
[0140] The synthesis of embodiment 1—2-bromo-4-fluoro-5-methylphenol (2)
[0141] A solution of 4-fluoro-3-methylphenol (400 g, 3.17 mol) in 8 L of dichloromethane was cooled to -70 °C, and Br was added dropwise at -60 °C 2 (527g, 3.33mol), the reaction was continued for 2 hours, and no reaction raw material remained by monitoring the reaction. Add 1L saturated Na 2 S 2 o 3 Solution and 3L water, the reaction solution was slowly raised to room temperature, separated, extracted the aqueous phase with dichloromethane (1L*2), combined the organic layer, post-processed, concentrated to obtain 705g product, and obtained 533g product by beating with n-hexane, yield 82%.
[0142] NMR data 1 H NMR (300MHz, CDCl 3 ,ppm), δ7.10(d,1H),6.82(d,1H),2.19(s,3H).
Embodiment 2
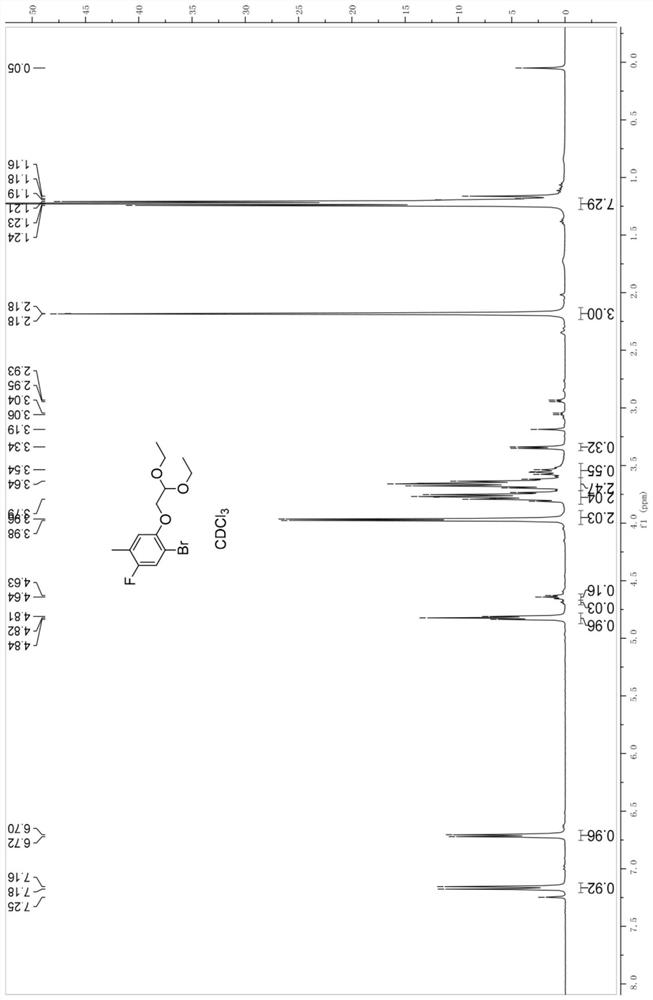
[0143] Synthesis of Embodiment 2—1-bromo-2-(2,2-diethoxyethoxy)-5-fluoro-4-toluene (4)
[0144] To a solution of 2-bromo-4-fluoro-3-methylphenol (530 g, 2.59 mol) in 2 L of DMF was added potassium carbonate (713 g, 5.17 mol) and 2-bromo-1,1-diethoxyethane (662 g, 3.36 mol). The mixture was heated to 120° C. and reacted for 16 h, and the reaction was monitored and no reaction raw material remained. The reaction was cooled to room temperature, the mixture was poured into 5L of water, then extracted with methyl tert-butyl ether (4L*3), the combined organic phase was washed with 4L of water and 4L of brine, dried and concentrated to obtain 902g of crude product.
[0145] NMR data 1 H NMR (300MHz, CDCl 3 ,ppm),δ7.16(d,1H),6.70(d,1H),4.82(t,1H),3.96(d,2H),3.79(m,2H),3.54(m,2H),2.18( s,3H), 1.21(t,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com