Multi-administration core-shell microneedle patch and preparation method thereof
A technology of micro-needle patch and core-shell micro, which is applied in the direction of micro-needles, pharmaceutical formulas, needles, etc., can solve the problems of restricted use, vaccine immune tolerance, etc., to increase the utilization rate, reduce the risk of biological hazards and disease transmission , improve the effect of treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1 to 5
[0054] The degradable drug-loaded microneedle patch products 1 to 5 of the present invention were prepared by the following general preparation process. The general preparation method comprises the following steps:
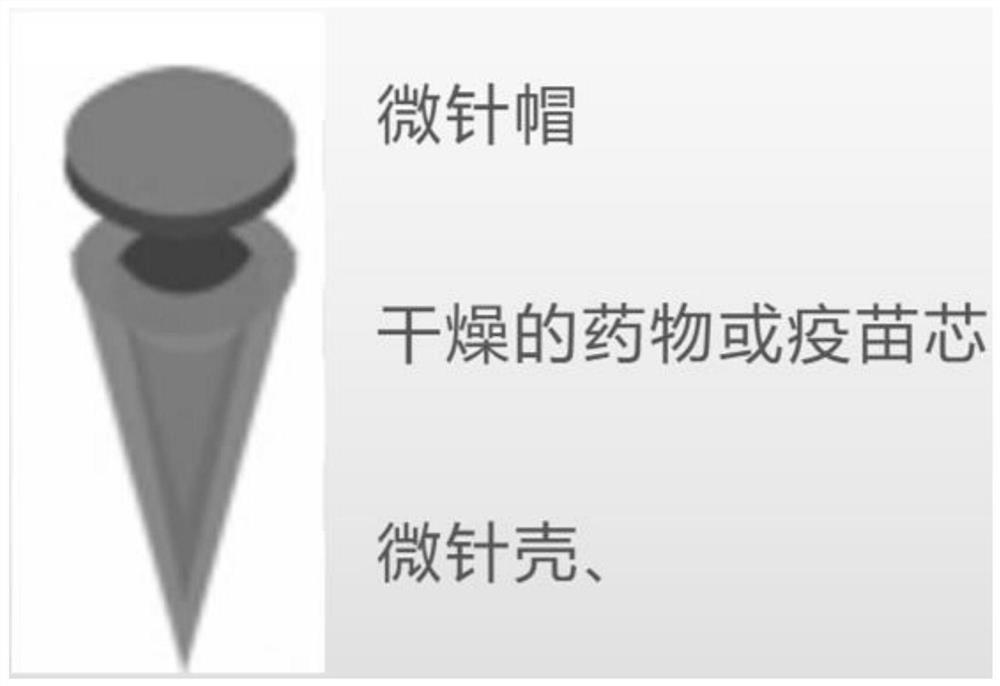
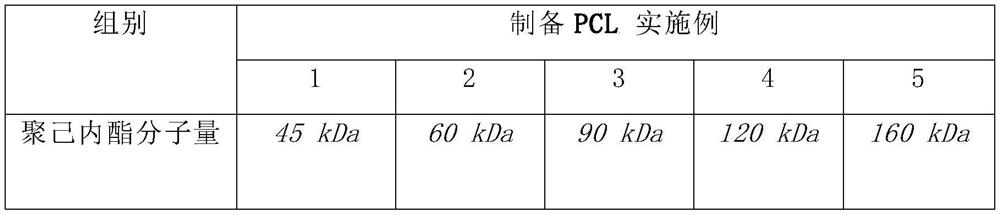
[0055] The microneedle shell and microneedle cap are made of biodegradable polymer, namely polycaprolactone (PCL). Preparation of PCL biodegradable polymer core-shell microneedles with different molecular weights.
[0056] Preparation of control samples:
[0057] Control samples were prepared using the same procedure as the general preparation procedure described above.
[0058] Table 1: Preparation of Control Samples and Preparation Examples 1 to 5
[0059]
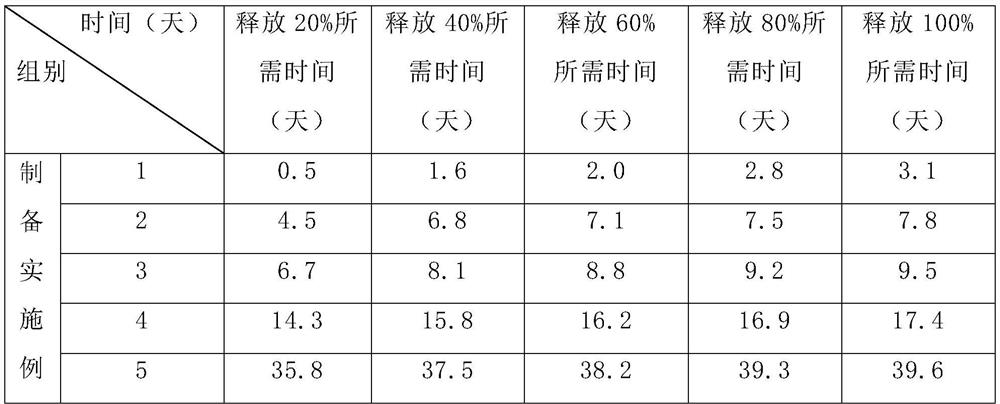
[0060] In vitro delayed burst release embodiment of core-shell microneedles
[0061] The specific operation steps are as follows: PCL biodegradable polymer core-shell microneedles with different molecular weights are prepared. We placed these microneedles containing fluorescent dyes as vaccine models...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Height | aaaaa | aaaaa |
| Core diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com