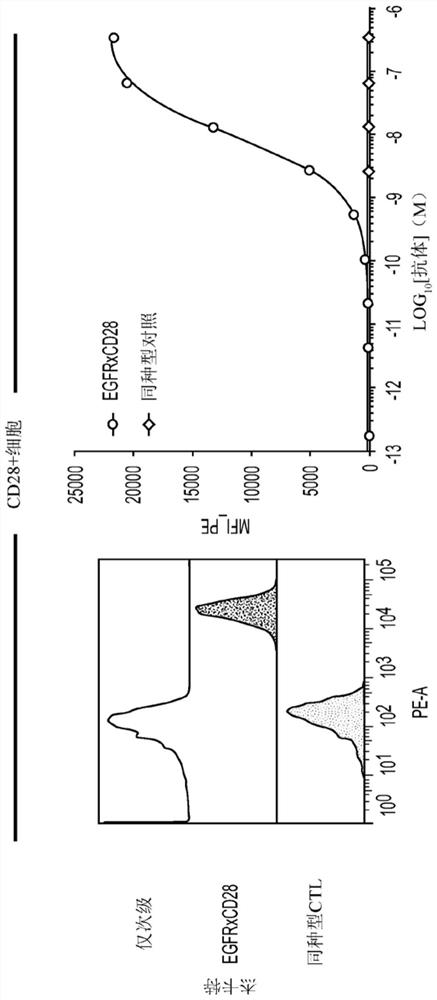
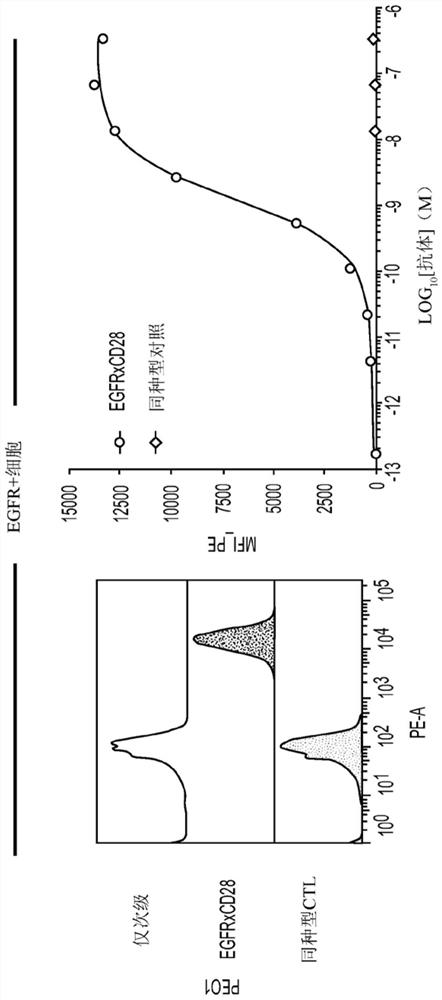
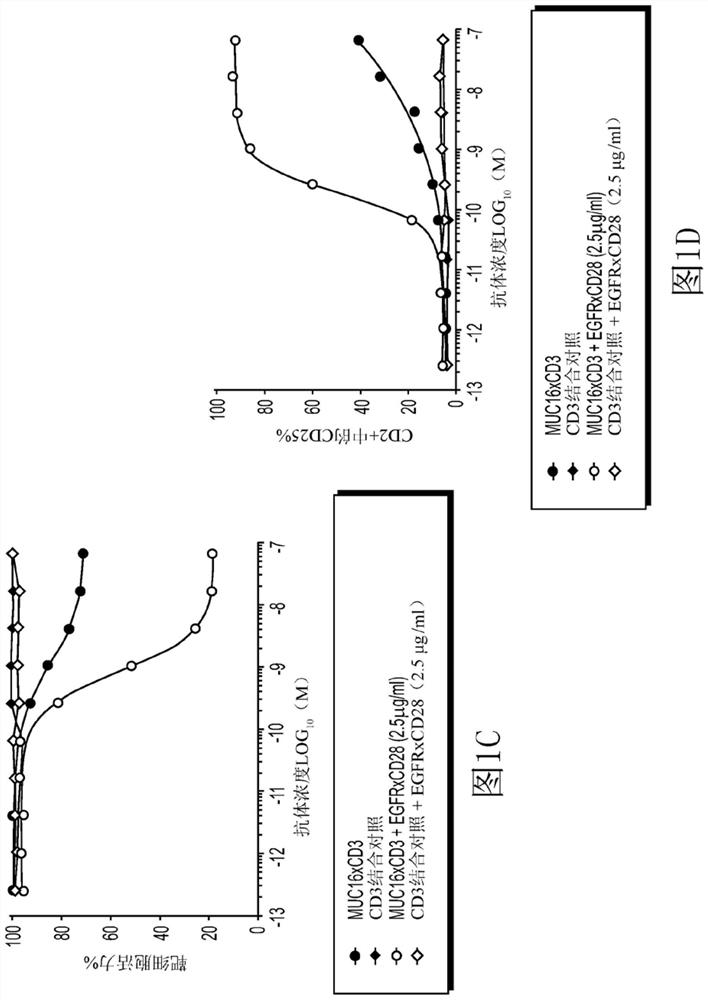
EGFRxCD28 MULTISPECIFIC ANTIBODIES
A multi-specific and bi-specific technology, applied in the direction of antibodies, antibody medical components, specific peptides, etc., can solve the problem of insufficient tumor clearance and anti-tumor response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0353] Preparation of antigen-binding domains and construction of bispecific molecules
[0354] Antigen-binding domains specific for a particular antigen can be prepared by any antibody production technique known in the art. Once obtained, two different antigen-binding domains specific for two different antigens (eg, CD28 and EGFR) can be suitably arranged relative to each other to generate bispecific antigen-binding molecules of the invention using conventional methods. (A discussion of exemplary bispecific antibody formats that can be used to construct bispecific antigen binding molecules of the invention is provided elsewhere herein). In certain embodiments, one or more of the individual components (e.g., heavy and light chains) of the multispecific antigen binding molecules of the invention are derived from chimeric antibodies, humanized antibodies, or Fully human antibody. Methods for preparing such antibodies are well known in the art. For example, VELOCIMMUNE can be ...
example
[0414] The following examples are presented to provide those of ordinary skill in the art with a complete disclosure and description of how to make and use the methods and compositions of the invention, and are not intended to limit the scope of what the inventors regard as their invention.
example 1
[0415] Example 1: Construction of anti-EGFRxCD28 antibody
[0416] Production of anti-EGFR antibodies
[0417] By directly administering an EGFR-expressing cell line (A431) with an adjuvant to stimulate an immune response to a DNA encoding a human immunoglobulin heavy-chain variable region and a universal light-chain variable region mice to obtain anti-EGFR antibodies. That is, antibodies produced in this mouse have different heavy chain variable regions but substantially identical light chain variable regions.
[0418] Antibody immune responses were monitored by EGFR-specific immunoassay. When the desired immune response is achieved, anti-EGFR antibodies are isolated directly from antigen-positive B cells without fusion with myeloma cells, as described in US7,582,298.
[0419] Table 1 shows the amino acid sequence identifiers of the heavy and light chain variable regions and CDRs of selected anti-EGFR antibodies of the invention. The corresponding nucleic acid sequence ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com