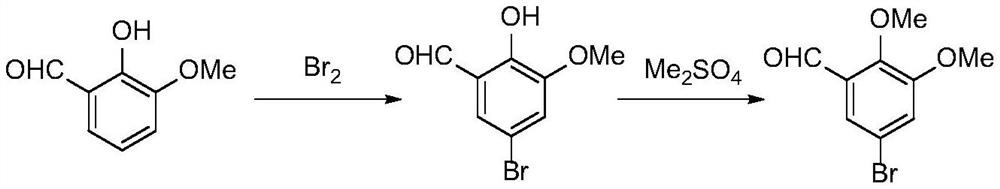
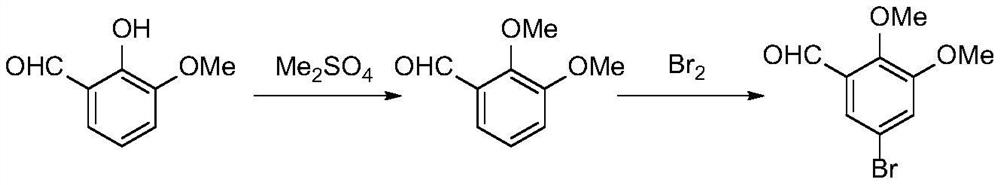
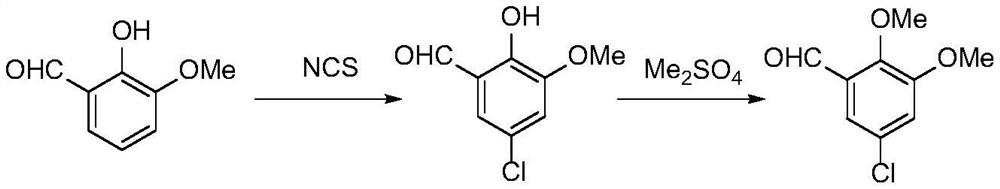
The synthetic method of 5-halogenated o-veratraldehyde
A technology of ortho-veratraldehyde and a synthesis method, which is applied in chemical instruments and methods, preparation of carbon-based compounds, preparation of organic compounds, etc., can solve the problems of high labor protection cost, limited source of ortho-vanillin, and high price. , to avoid toxic and harmful raw materials, reduce protection costs, and achieve the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] 1.1 Preparation of 4-bromoguaiacol
[0063] Under argon protection, guaiacol (25 g, 201 mmol) was uniformly dispersed in 125 mL of chloroform, and then the reaction solution was pre-cooled at -5 °C for 10 minutes, and bromine (10.3 ml, 201 mmol) was slowly added dropwise thereto. chloroform (75mL) solution, try to keep the system colorless. After the dripping was completed, the reaction was moved to room temperature for 1 hour, and the reaction was detected by TLC dot plate. With 150mL Sat.NaHSO 3 (aq) Quenching the reaction, separating the liquids in a separating funnel, extracting the aqueous layer three times with chloroform, combining the organic phases, backwashing once with saturated brine, drying over anhydrous sodium sulfate and spin-dried to obtain pale yellow crystals Crude. Under reduced pressure distillation (97-100°C / 2mmHg, Lit. 119-120°C / 5mmHg), 4-bromoguaiacol (40g, 94%, mp=31-32°C) was obtained.
[0064] 1 H NMR (400MHz, Chloroform-d) δ 6.99 (dd, J=...
Embodiment 2
[0081] 2.1 Preparation of 4-chloroguaiacol
[0082] Under an ice-salt bath under argon protection (-5°C), guaiacol (12.4 g, 100 mmol) was uniformly dispersed in 50 mL of chloroform, and sulfonyl chloride (13 mL, 160 mmol) was slowly added dropwise thereto. After the dripping was completed, it was moved to 60°C for reflux reaction for 24h, and the reaction was monitored by TLC spot plate. Under ice bath, use 50mL Sat.Na 2 S 2 O 3 The solution was quenched and the reaction was separated in a separating funnel. The aqueous layer was extracted three times with chloroform. The organic phases were combined, backwashed once with saturated brine, dried over anhydrous sodium sulfate and spin-dried to obtain 16.7 g of a brownish-yellow liquid. Crude. After distillation under reduced pressure (82-90°C / 2mmHg, lit.130°C / 7.5mmHg), light yellow oily liquid 4-chloroguaiacol (12.7g, 80%) was obtained with a content of 87% (GC-MS).
[0083] 1 H NMR (400MHz, Chloroform-d) δ6.84(s,3H), 5.61...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com