3-acylamino-N-arylbenzamide compound and application thereof
An aryl benzamide and amide-based technology, applied in the field of medicine, can solve problems such as sequelae, achieve good therapeutic effects, inhibit the level of epileptic seizures, and prolong the effect of seizure latency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
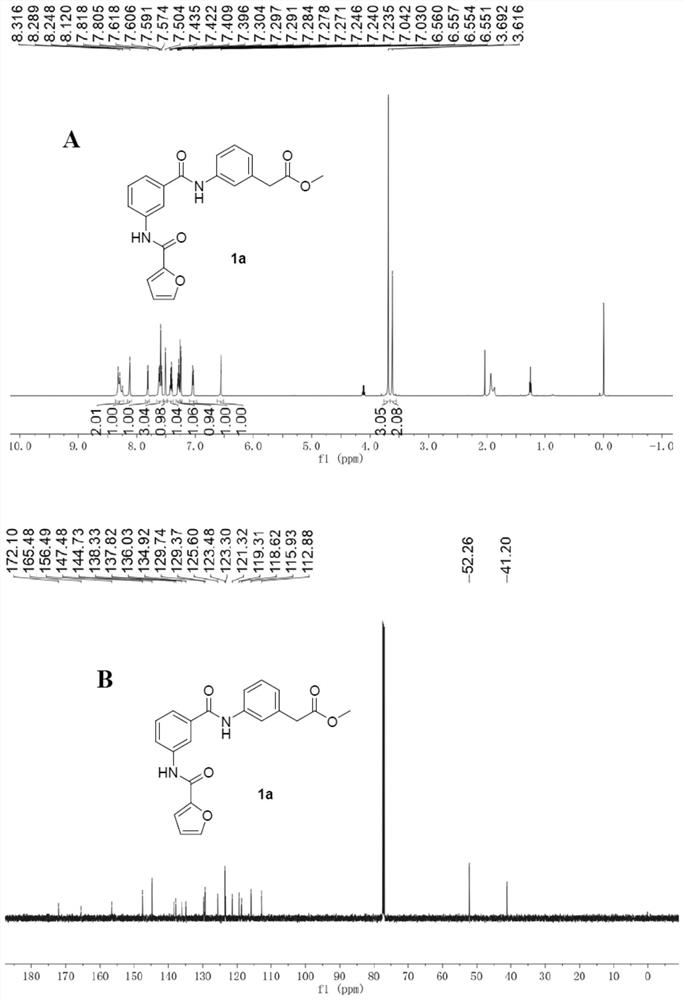
[0147] Example 1 3-(3-(furan-2-carboxamido)benzamido)methyl phenylacetate
[0148]
[0149] Step A: Add 3-nitrobenzaldehyde (2) (3.02 g, 20 mmol) into a 100 ml one-necked bottle, and add 20 ml of methanol to dissolve it. The reaction solution was cooled to below 0°C, and sodium borohydride (2.27g, 60mmol) was added in batches, and reacted at 0°C for 10min after the addition was complete. After the reaction, the methanol was removed under reduced pressure, the residue was added with water and extracted three times with 20ml ethyl acetate, the organic phase was washed three times with 10mL saturated brine, the organic phase was dried with anhydrous sodium sulfate and the ethyl acetate was removed under reduced pressure to obtain a colorless The oily 3-nitrobenzyl alcohol (3) is directly subjected to the next reaction.
[0150] Step B: Add the 3-nitrobenzyl alcohol (3) obtained in Step A into a 100ml one-necked bottle, and add 30ml of dichloromethane to dissolve it. The reac...
Embodiment 2
[0157] Example 2 Methyl 3-(3-(thiophene-2-carboxamido)benzamido)phenylacetate
[0158]
[0159] Compound 1b NMR data 1 HNMR (400MHz, DMSO-d 6 )δ10.45(s,1H),10.30(s,1H),8.25(s,1H),8.08(s,1H),8.00(d,J=8.0Hz,1H),7.89(d,J=4.8 Hz, 1H), 7.70(m, 3H), 7.52(t, J=8.0Hz, 1H), 7.31(t, J=7.6Hz, 1H), 7.25(t, J=3.2Hz, 1H), 7.01( d,J=7.6Hz,1H),3.69(s,2H),3.63(s,3H); 13 CNMR (100MHz, DMSO-d 6 )δ171.5,165.5,160.0,139.9,139.2,138.9,135.6,134.8,132.1,129.3,128.7,128.6,128.2,124.7,123.3,122.7,121.1,119.9,118.9,51.7,zIMS.4: =395[M+H + ].
Embodiment 3
[0160] Example 3 Methyl 3-(3-(5-chlorothiophene-2-carboxamido)benzamido)phenylacetate
[0161]
[0162] Compound 1c NMR data 1 HNMR (600MHz, DMSO-d 6 )δ10.50(s,1H),10.28(s,1H),8.21(s,1H),7.96(s,2H),7.69(s,3H),7.50(s,1H),7.28(m,2H ),7.00(s,1H),3.64(m,5H); 13 CNMR (150MHz, DMSO-d 6 )δ172.01,165.86,159.47,139.69,139.38,139.08,136.11,135.25,134.69,129.82,129.26,129.12,128.84,125.24,123.78,123.41,121.62,120.39,119.46,52.20,40.84;ESI-MS:m / z =430[M+H + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com