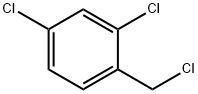
Synthesis method of 2, 4-dichlorobenzyl chloride
A dichlorobenzyl chloride and synthetic method technology, applied in the field of fine chemical organic synthesis, can solve the problems of low conversion rate, high preparation cost, complicated preparation process, etc., and achieve the effect of simplifying the rectification procedure, simplifying the procedure and reducing the cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] (1) Heat 16.1g of 2,4-dichlorotoluene to 100°C and add it to the chlorination reaction kettle, add the catalyst 0.016g of azobisisobutyronitrile and 0.012g of triethanolamine, and slowly introduce chlorine gas 8.17g under light conditions g, while raising the temperature to 125°C, reacting for 3.5 hours to generate 2,4-dichlorobenzyl chloride solution, and using a graphite absorber to recover the HCl gas generated during the reaction;
[0022] (2) The 2,4-dichlorobenzyl chloride solution obtained in step (1) was subjected to vacuum distillation, and the product 2,4-dichlorobenzyl chloride 18.96g and 2,4-dichlorobenzylidene dichloride were collected Chlorine 0.30g.
Embodiment 2
[0024] (1) Heat 16.1 g of 2,4-dichlorotoluene to 90°C and put it into the chlorination reaction kettle, add the catalyst 0.013g of azobisisobutyronitrile and 0.0125g of triethanolamine, and slowly introduce chlorine gas at 7.81 g under light conditions g, while raising the temperature to 120°C and reacting for 4 hours to generate 2,4-dichlorobenzyl chloride solution, while using a graphite absorber to recover the HCl gas generated during the reaction;
[0025] (2) The 2,4-dichlorobenzyl chloride solution obtained in step (1) was rectified under reduced pressure, and the product 2,4-dichlorobenzyl chloride 18.86 g and 2,4-dichlorobenzyl chloride were collected. Chlorine 0.35 g.
Embodiment 3
[0027] (1) Heat 16.1 g of 2,4-dichlorotoluene to 80°C and put it into the chlorination reaction kettle, add the catalyst 0.019 g of azobisisobutyronitrile and 0.01 g of triethanolamine, and slowly introduce chlorine gas of 8.0 g under light conditions g, while raising the temperature to 130°C, reacting for 3 hours to generate 2,4-dichlorobenzyl chloride solution, and using a graphite absorber to recover the HCl gas generated during the reaction;
[0028] (2) The 2,4-dichlorobenzyl chloride solution obtained in step (1) was rectified under reduced pressure, and the product 2,4-dichlorobenzyl chloride 18.60 g and 2,4-dichlorobenzyl chloride were collected. Chlorine 0.48 g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com