Target particle size emulsified cross-linked sodium hyaluronate particle enrichment and cross-linking agent cleaning method
A cross-linked hyaluronic acid and sodium hyaluronate technology, which is applied in the field of medical materials, can solve the problem of difficult cleaning of cross-linking agents, wide particle size distribution of sodium hyaluronate particles, and restrictions on the application of emulsified cross-linked sodium hyaluronate particles and other issues to achieve the effect of saving materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1, collection of cross-linked sodium hyaluronate particles of target particle size
[0028] 1) Add 500ml of absolute ethanol to 10g of emulsified cross-linked sodium hyaluronate particles, and stir until the particles are evenly suspended;
[0029] 2) Stop stirring, let stand to settle, and after 30 minutes, take the upper layer suspension, transfer it to a negative pressure filter device, and vacuum filter to obtain sodium hyaluronate particles;
[0030] 3) Repeat the absolute ethanol collection process 3 times;
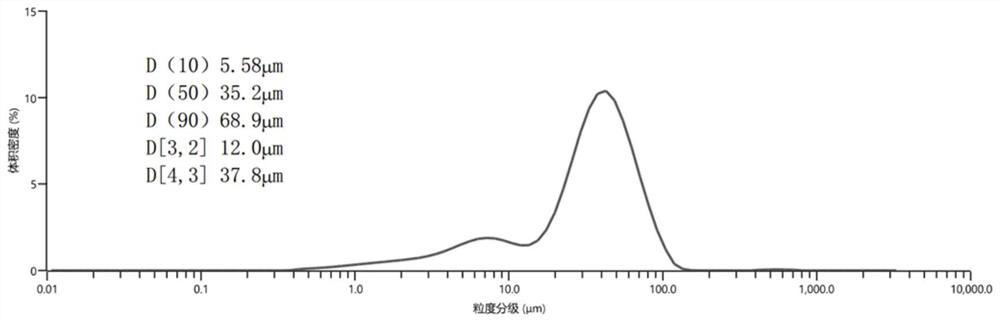
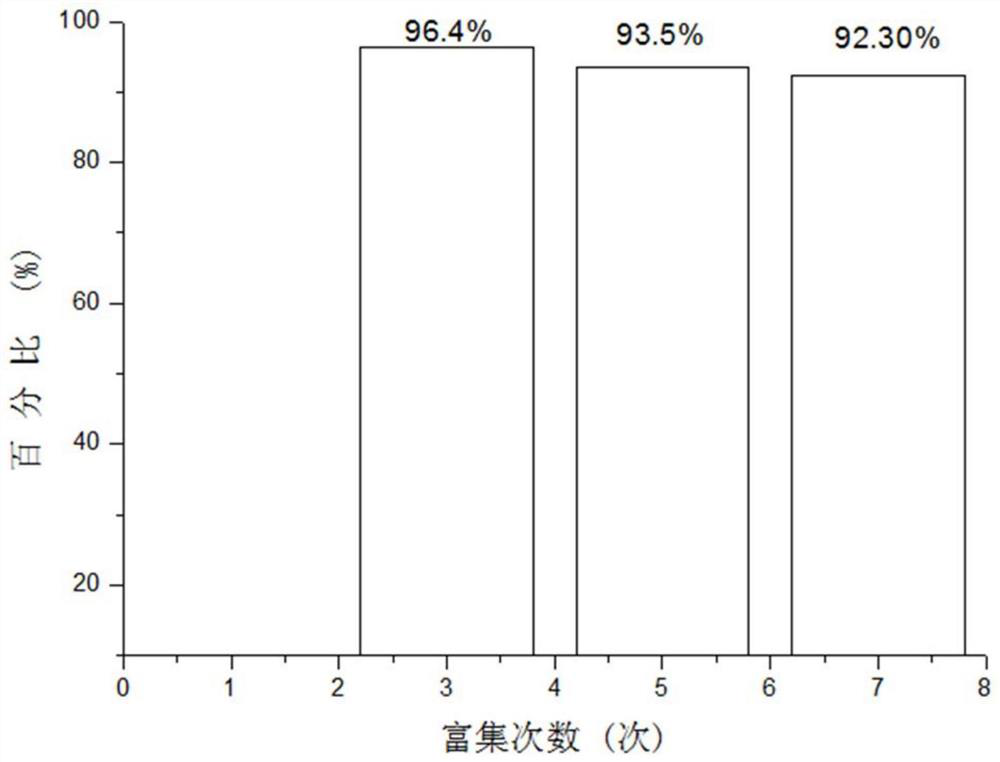
[0031] 4) The collected cross-linked sodium hyaluronate particles were dried in vacuum at 60°C for 12 hours, the collected particles were weighed, and the mass of the enriched particles was recorded as 9.64g, accounting for 96.4% of the total mass (see figure 2 ). Malvern mastersizer3000 was used to detect the particle size distribution of the target sample, and the test results are shown in the appendix figure 1 ,Depend on figure 1 It can be see...
Embodiment 2
[0032] Embodiment 2, collection of cross-linked sodium hyaluronate microparticles of target particle size
[0033] 1) Add 400ml of absolute ethanol to 8.0g of emulsified cross-linked sodium hyaluronate particles, and stir until the particles are uniformly suspended;
[0034] 2) Stop stirring, let stand to settle, and after 30 minutes, take the upper layer suspension, transfer it to a negative pressure filter device, and vacuum filter to obtain sodium hyaluronate particles;
[0035] 3) Repeat the absolute ethanol collection process 5 times,
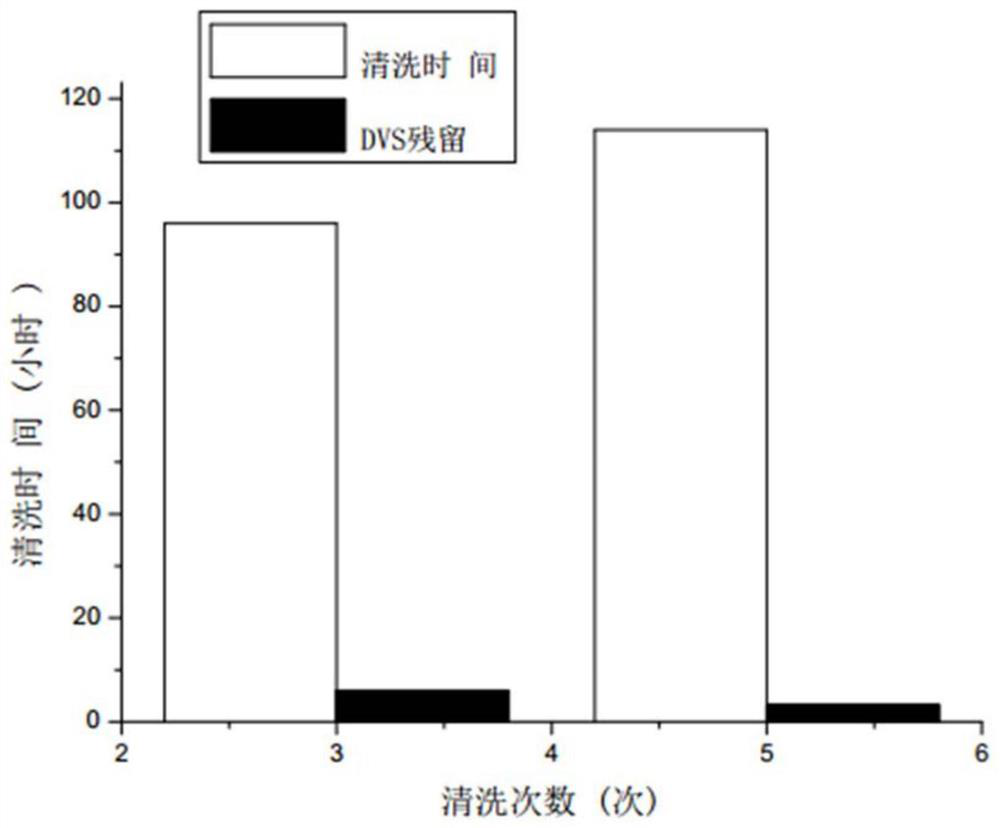
[0036] 4) The collected cross-linked sodium hyaluronate particles were vacuum-dried at 45°C for 12 hours, the collected particles were weighed, and the mass of the enriched particles was recorded as 9.35g, accounting for 93.5% of the total mass (see figure 2 ). The residual amount of DVS in the sample detected by gas chromatography was 40.7μg / g.
Embodiment 3
[0037] Embodiment 3, collection of cross-linked sodium hyaluronate microparticles of target particle size
[0038] 1) Add 240ml of absolute ethanol to 6.0g of emulsified cross-linked sodium hyaluronate particles, and stir until the particles are evenly suspended;
[0039] 2) Stop stirring, let stand to settle, and after 30 minutes, take the upper layer suspension, transfer it to a negative pressure filter device, and vacuum filter to obtain sodium hyaluronate particles;
[0040] 3) Repeat the absolute ethanol collection process 6 times,
[0041] 4) The collected cross-linked sodium hyaluronate particles were then vacuum-dried at 75° C. for 12 hours, collected and weighed, and the mass of the enriched particles was recorded as 9.35 g, accounting for 93.5% of the total mass (see figure 2 ). The residual amount of DVS in the sample detected by gas chromatography was 39.5 μg / g.
[0042]The density of absolute ethanol matches the target particle size, so that the cross-linked s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com