Skin external medicament as well as composition and preparation method thereof
A composition for external use and the technology of the composition, applied in the field of medicine, can solve the problems of unsteady release, easy deterioration, and unstable storage process of imidazole external preparations, so as to avoid fluctuations in blood drug concentration, reduce irritation, and inhibit deterioration. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] (1) Prepare the composition
[0083] (1) liposome group, including:
[0084] Phospholipids: distearoylphosphatidylcholine, 6.5 parts by weight;
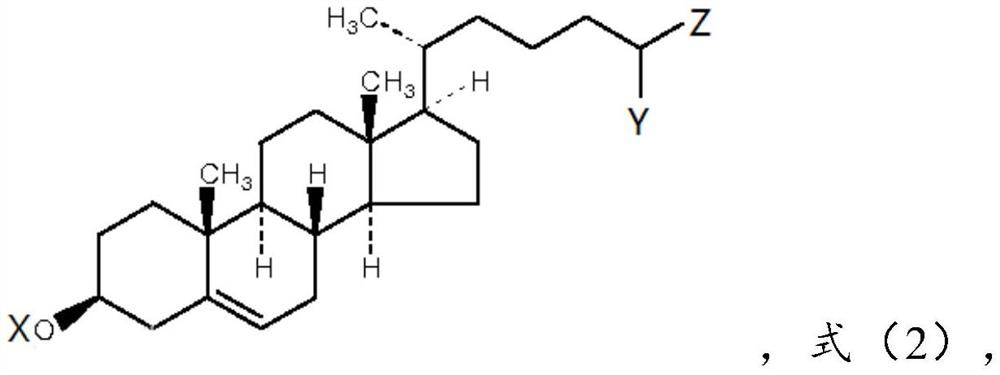
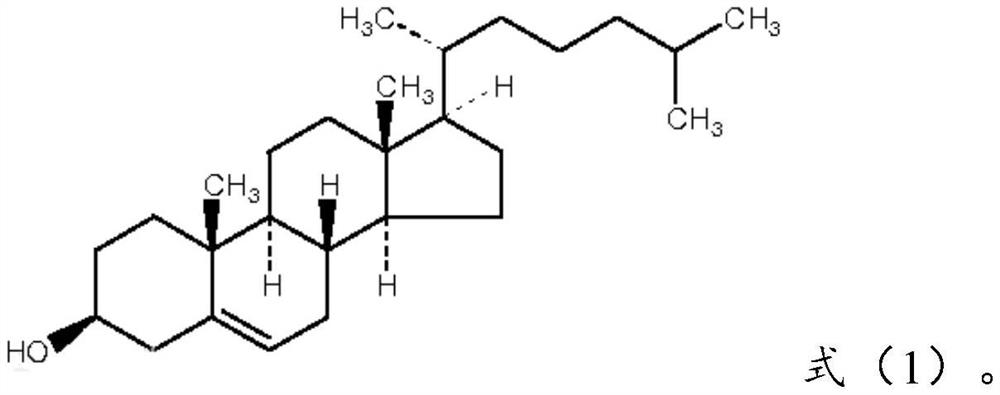
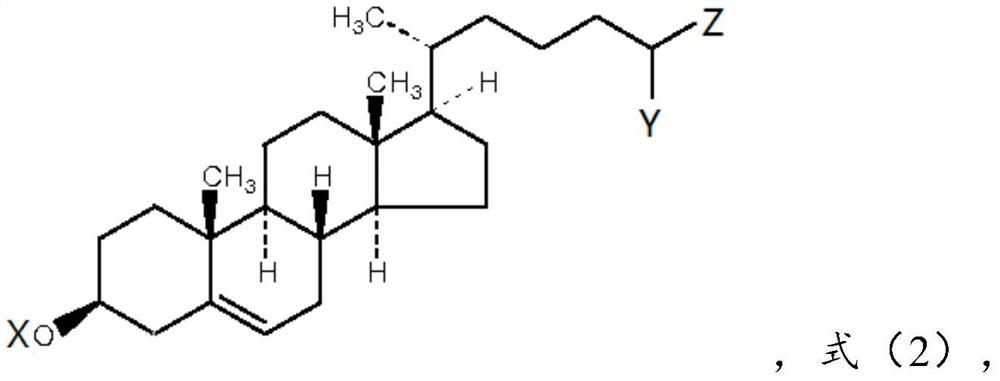
[0085] Cholesterol: compound of formula (1), 0.7 parts by weight;
[0086] Cholesterol derivatives: compounds of formula (2-1), wherein X is H, Y is methyl, Z is -COOH, 2.7 parts by weight;
[0087] The first penetration enhancer: isopropyl myristate, 1 part by weight;
[0088] (2) matrix group, including:
[0089] Fatty acid ester: glycerin fatty acid ester (fatty acid part is a saturated C18 carbon chain), 50 parts by weight;
[0090] Water-based gel compound: hydroxyethyl cellulose, 20 parts by weight;
[0091] The second penetration enhancer: isopropyl myristate, 0.1 parts by weight;
[0092] (3) Drugs:
[0093] The first drug: luliconazole, 6 parts by weight;
[0094] The second drug: luliconazole, 1 part by weight.
[0095] (4) Other components, including:
[0096] Solvent: absolute ethanol;
[0097] Buffer so...
Embodiment 2
[0104] (1) Prepare the composition
[0105](1) liposome group, including:
[0106] Phospholipids: distearoylphosphatidylcholine, 6 parts by weight;
[0107] Cholesterol: compound of formula (1), 0.8 parts by weight;
[0108] Derivatives of cholesterol: compounds of formula (2-2), wherein X is ethyl, Y is H, and Z is -SO 3 H, 3.2 parts by weight;
[0109] The first penetration enhancer: isopropyl myristate, 1 part by weight;
[0110] (2) matrix group, including:
[0111] Fatty acid ester: sucrose fatty acid ester (fatty acid part is a saturated C12 carbon chain), 32 parts by weight;
[0112] Water-based gel compound: hydroxypropyl cellulose, 8 parts by weight;
[0113] The second penetration enhancer: isopropyl myristate, 0.1 parts by weight;
[0114] (3) Drugs:
[0115] The first drug: levconazole, 5 parts by weight;
[0116] Second drug: None.
[0117] (4) Other components, including:
[0118] Solvent: absolute ethanol;
[0119] Buffer solution: Phosphate buffer s...
Embodiment 3
[0122] (1) Prepare the composition
[0123] (1) liposome group, including:
[0124] Phospholipids: distearoylphosphatidylcholine, 7 parts by weight;
[0125] Cholesterol: compound of formula (1), 0.7 parts by weight;
[0126] Cholesterol derivatives: compounds of formula (2-3), wherein X is H, Y is -COOH, and Z is -SO 3 H, 2.3 parts by weight;
[0127] The first penetration enhancer: isopropyl myristate, 1 part by weight;
[0128] (2) matrix group, including:
[0129] Fatty acid ester: xylitol fatty acid ester (fatty acid part is a saturated C16 carbon chain), 54 parts by weight;
[0130] Aqueous gel compound: hypromellose, 46 parts by weight;
[0131] The second penetration enhancer: isopropyl myristate, 0.1 parts by weight;
[0132] (3) Drugs:
[0133] The first drug: Praconazole, 6 parts by weight;
[0134] The second drug: praconazole, 1 part by weight.
[0135] (4) Other components, including:
[0136] Solvent: absolute ethanol;
[0137] Buffer solution: Phosp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com