Bispecific t-cell engager with cleavable cytokines for targeted immunotherapy
A non-immunogenic, compound technology that can be used in drug combinations, medical preparations with non-active ingredients, anti-tumor drugs, etc., and can solve the problems of side effects such as T cell depletion and depletion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
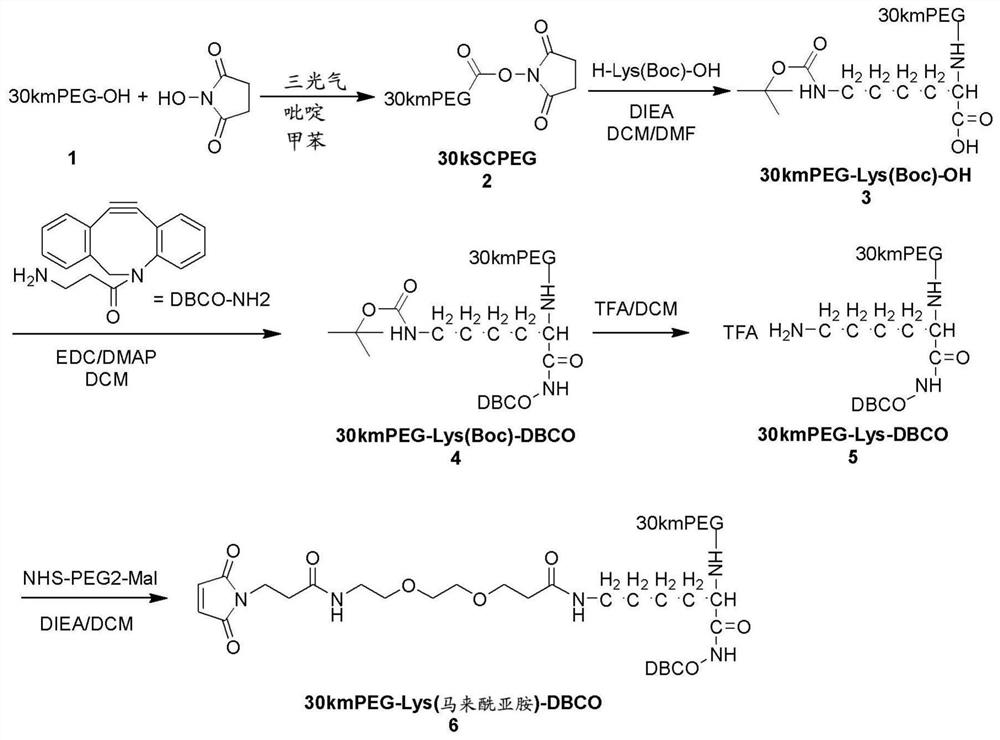
[0263] Embodiment 1: the preparation of 30kmPEG-Lys (maleimide)-DBCO ( figure 1 )
[0264] Preparation of 30kmSC-PEG (Compound 2):
[0265] 25 g of 30 kmPEG-OH (MW=30000, 1 eq.) was azeotroped with 360 mL of reagent toluene for 2 hours to remove 75 mL of toluene / water. After constant boiling, the solution was cooled to 45 to 50°C. 166 mg of triphosgene (0.67 eq.) was added to PEG, followed by 131.8 mg of anhydrous pyridine (2 eq.). The reaction was stirred at 50°C for 3 hours. Then 239.8 mg of N-hydroxysuccinimide (2.5 eq.) were added, followed by 164.8 g of anhydrous pyridine (2.5 eq.). The reaction mixture was stirred overnight at 50 °C under nitrogen. Pyridinium salts were filtered. The solvent was removed using a Rotavapor and the residue was recrystallized from 2-propanol. The isolated product was dried in a vacuum oven at 40 °C to obtain 23 g of 30 kmSC-PEG.
[0266] Preparation of 30kmPEG-Lys(Boc)-OH (Compound 3):
[0267] 369 mg of H-lys(boc)-OH (3 eq.), 646.5...
Embodiment 2
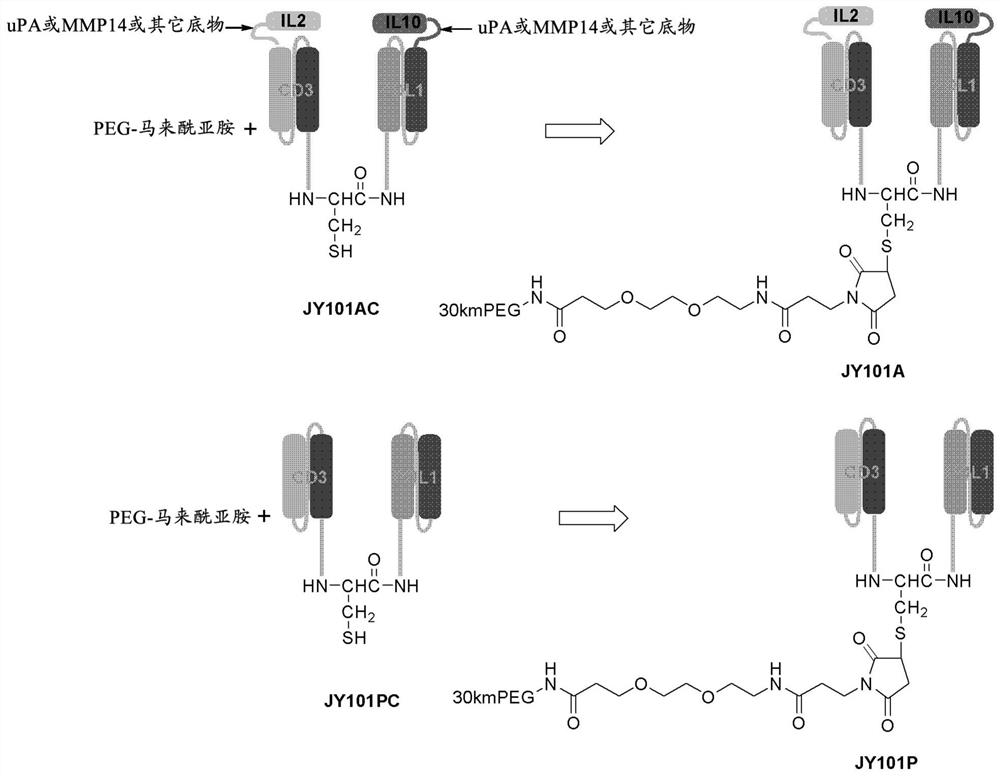
[0274] Example 2: Preparation of SCACD3IL2 and SCAPDL1IL10
[0275] The two cytokine-capped single-chain antibody fragment proteins in the present invention are prepared according to the highlighted marks in formula Ib. A 1 -L 3 -C 1 (A 1 -L 3 -C 1 ) of the first protein consists of IL2V+uPA substrate+MMP14 substrate+anti-CD3 (SCACD3IL2), the second protein-A 2 -L 4 -C 2 (A 2 -L 4 -C 2 ) is IL10+uPA+MMP14 substrate+anti-PDL1 (SCAPDL1IL10). Both proteins were produced by recombinant DNA technology in Chinese hamster ovary (CHO) cells knocked out of GS using the pD2531nt-HDP expression vector containing the GS gene (both cell line and vector licensed from Horizon Discovery, Inc) . DNA encoding the first protein (SCACD3IL2) and the second protein (SCAPDL1IL10) were synthesized and cloned into pD2531nt-HDP expression vector, and transfected into CHO-GS(- / -) cells. Stable cell lines with high productivity were obtained by culturing cells in medium containing the GS in...
Embodiment 3
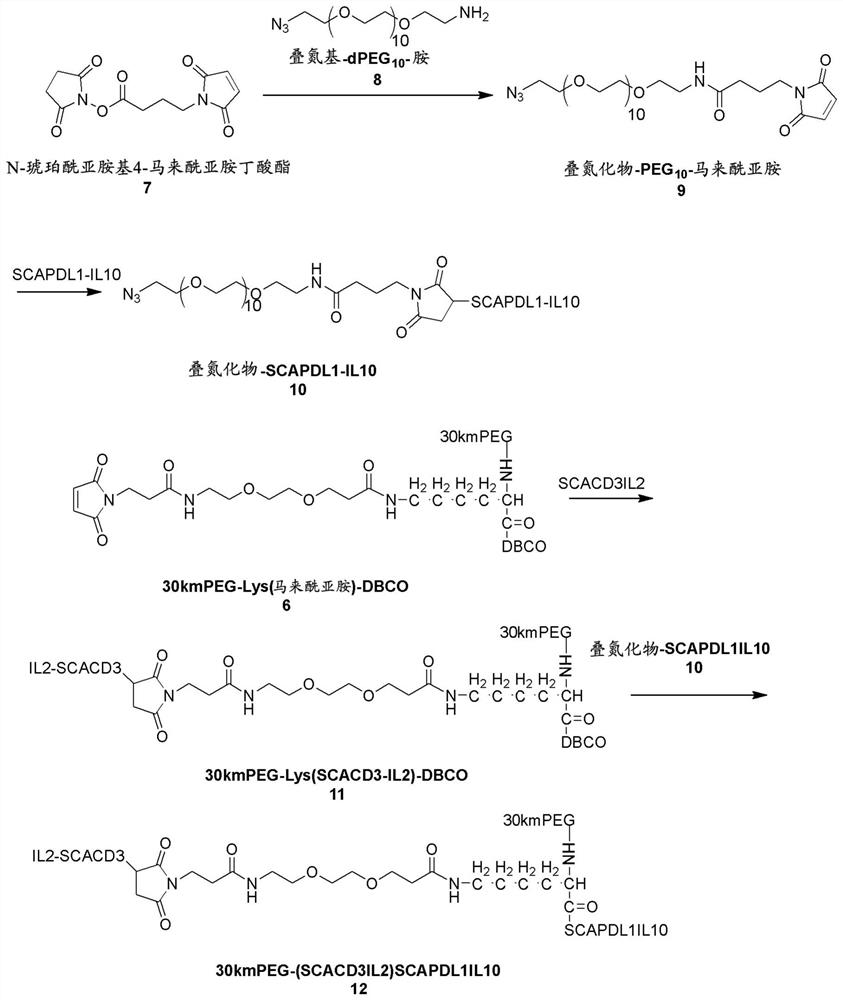
[0280] Example 3: Preparation of 30kmPEG-(SCAPDL1IL10)SCACD3IL2( figure 2 )
[0281] Preparation of compound 9:
[0282] N-Succinimidyl 4-maleimidobutyrate (1 eq.) was reacted with azido-dPEG10-amine (1.5 eq.) in DMSO at room temperature for 45 minutes. The obtained compound 9 azide-PEG10-maleimide was directly used in the next step without further purification.
[0283] Preparation of Compound 10:
[0284] TCEP-HCl (Product No. 580560, Sigma-Aldrich) was added to SCAPDL1IL10 (5-10 mg / mL) at a final concentration of 2-10 mM in 200 mM phosphate buffer (pH 6.8). The reaction was mixed well and left at room temperature for 30 minutes. Reduced SCAPDL1IL10 (1 eq.) was reacted with compound 9 (100 eq.) at room temperature for 1 hour. The reaction was quenched with 10 mM cystine for 10 min at room temperature. Excess compound 9 was removed by desalting column in PBS buffer. Fractions of desired compound 10 azide-SCAPDL1IL10 were pooled and concentrated to 5-10 mg / ml for the n...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com