Sustained-release anti-FcRn antibody or antigen binding fragment and application thereof
A technology for combining fragments and antibodies, which is applied in the field of slow-release anti-FcRn antibodies or antigen-binding fragments, treatment, prevention and/or diagnosis of FcRn-related diseases, and can solve the problem of short half-life of FcRn antibodies or Fc fragments, fast metabolism of antibody drugs, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1: Nanobody construction
[0044] Camels were immunized with antigens, peripheral blood mononuclear cells (PBMC) were isolated and total RNA was extracted for reverse transcription, and the variable domain of the heavy-chain of heavy chain antibody was amplified using the reverse transcription product as a template , VHH) and connected to the phage display vector, electroporated into Escherichia coli TG1 competent cells, and camel immune library was constructed.
[0045] Specifically, camels are immunized once every two weeks, a total of 4 times. Each injection of 0.8 mg HSA extracellular region recombinant protein (purchased from Hualan Bioengineering Co., Ltd., product batch number: 201405030), accompanied by Freund's complete / incomplete adjuvant (Sigma, F5881, F5506), was taken subcutaneously at multiple points The way of injection. Two weeks after each immunization, 1 mL of blood was collected to separate the serum, and the immunogen was used as the assay ...
Embodiment 2
[0047] Example 2: Construction of FcRn sustained release antibody
[0048] In this example, the HSA nanobody HzH4-3 and Hz2MG6m19 sequences obtained in Example 1 were connected to the C-terminus of the FcRn antibody Efgartigimod (Ef for short, the sequence is shown in SEQ ID NO: 9) to construct H4-3-Ef and m19 -Ef eukaryotic expression vector, and then transfected into eukaryotic cells for expression and purification to obtain slow-release antibodies against hFcRn H4-3-Ef and m19-Ef.
Embodiment 3
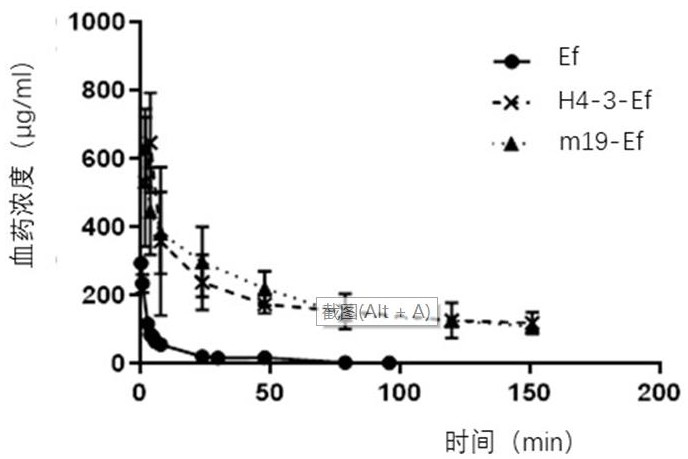
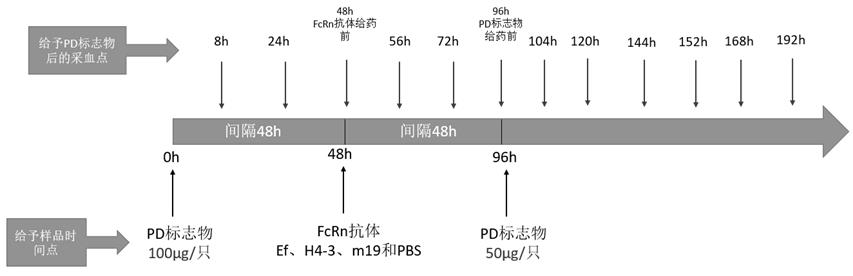
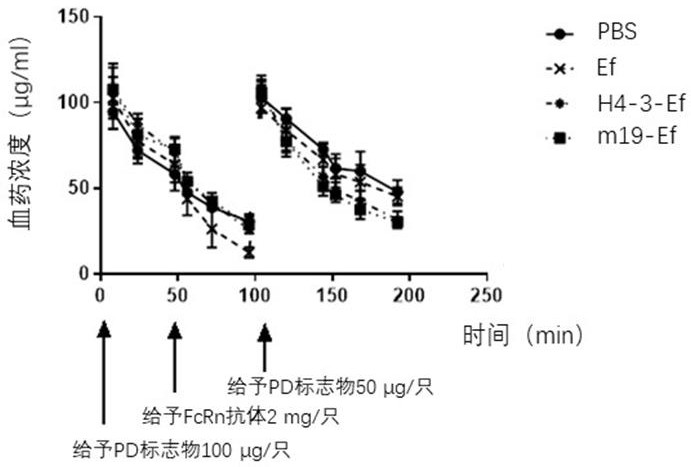
[0049] Example 3: ELISA method to detect the metabolism of H4-3-Ef and m19-Ef in HSA / FcRn double-transformed humanized mice
[0050] The experiment was conducted with hFcRn female 6-week-old transgenic mice, divided into 3 groups, 4 mice in each group, and the first two groups were intravenously injected with H4-3-Ef and m19-Ef, both at 300 μg / mouse. Then blood was collected by docking at 2h, 4h, 8h, 24h, 48h, 79h, 120h, 151h and 192h. Another group was injected with Ef at a rate of 300 μg / bird, and then blood was collected at 0.5h, 1h, 3h, 4h, 5h, 6h, 8h, 24h, 30h, 48h, 79h and 96h, and the serum was collected and stored at -20°C. save.
[0051] After all the blood collection is completed, the collected serum is tested by ELISA, and the steps are as follows:
[0052] 1) Coating anti-Fc Ab (Sigma, I2136) onto 96-well ELISA plate, 0.5 μg / mL, 100 μL / well, overnight at 4°C;
[0053] 2) After washing the plate 3 times with PBS, add 0.5% casein-PBS, block at 37°C for 60 min, and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com