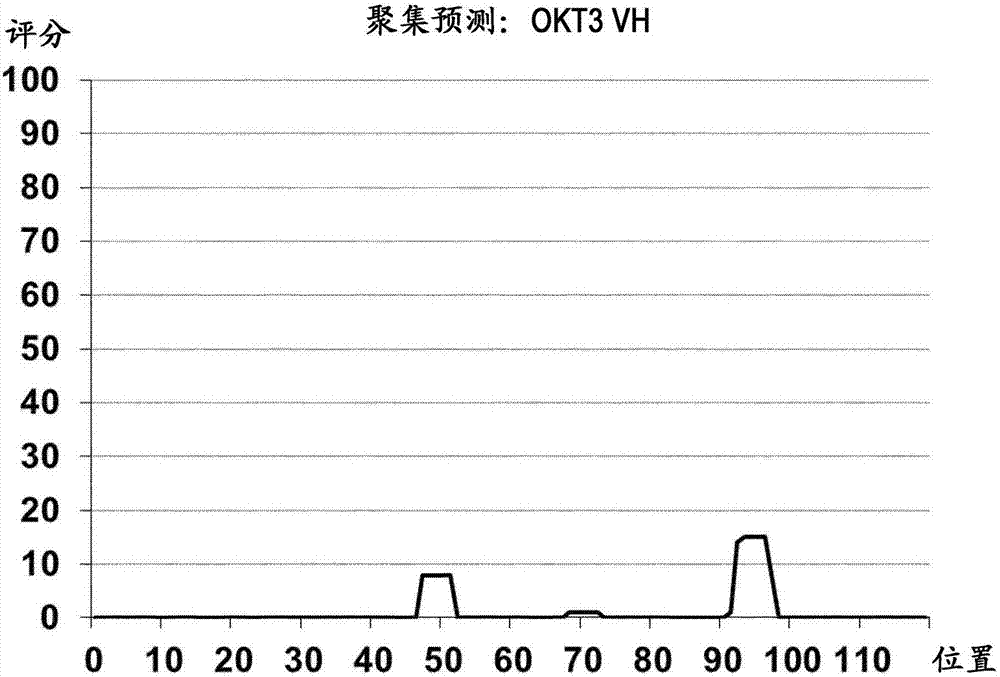
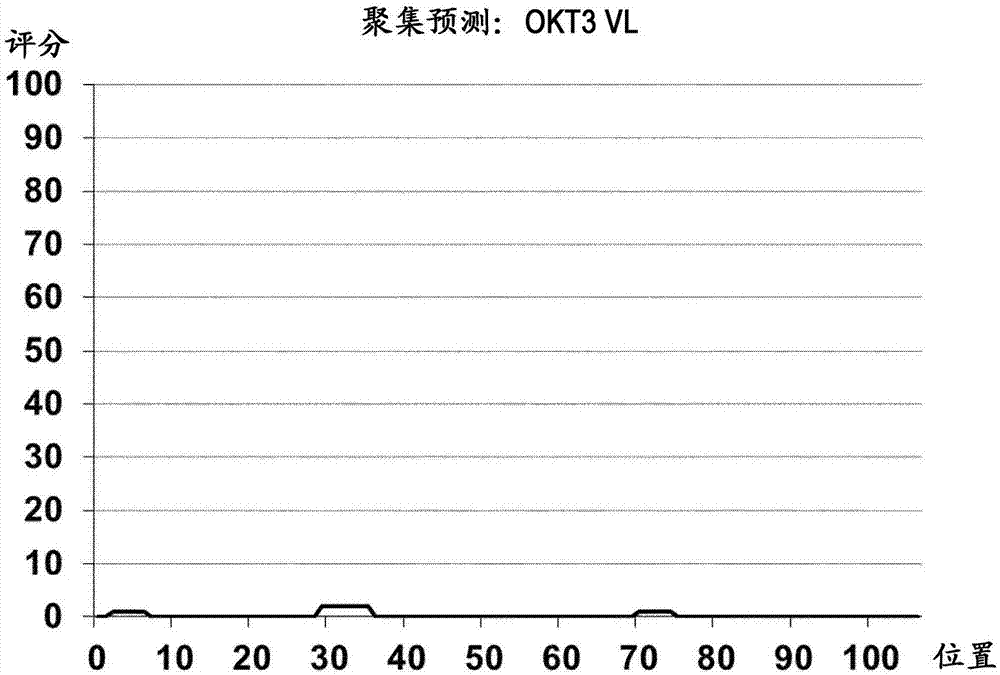
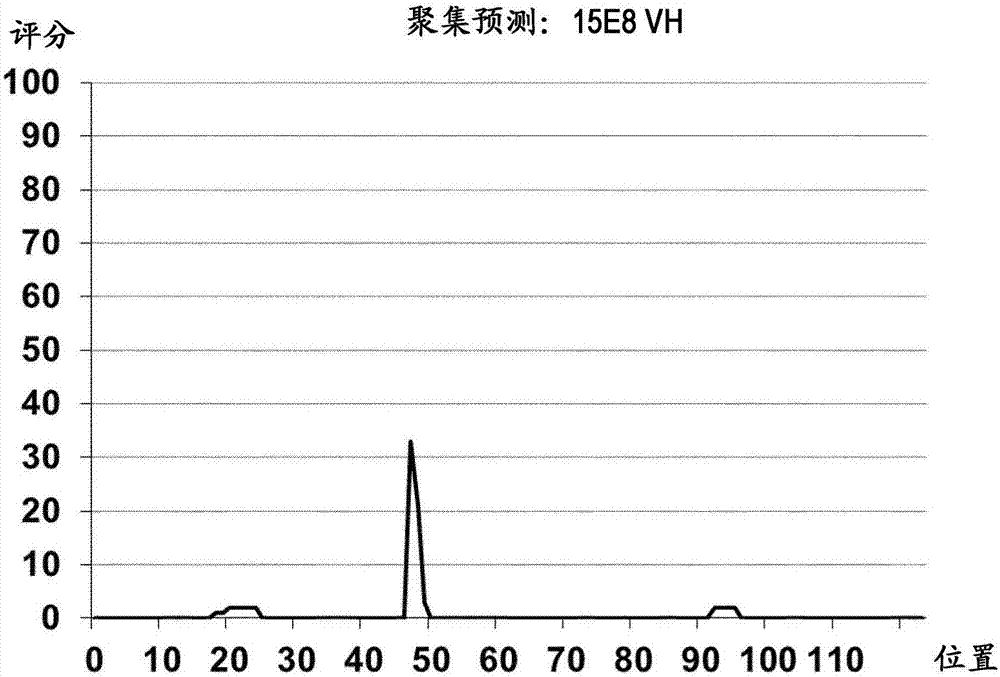
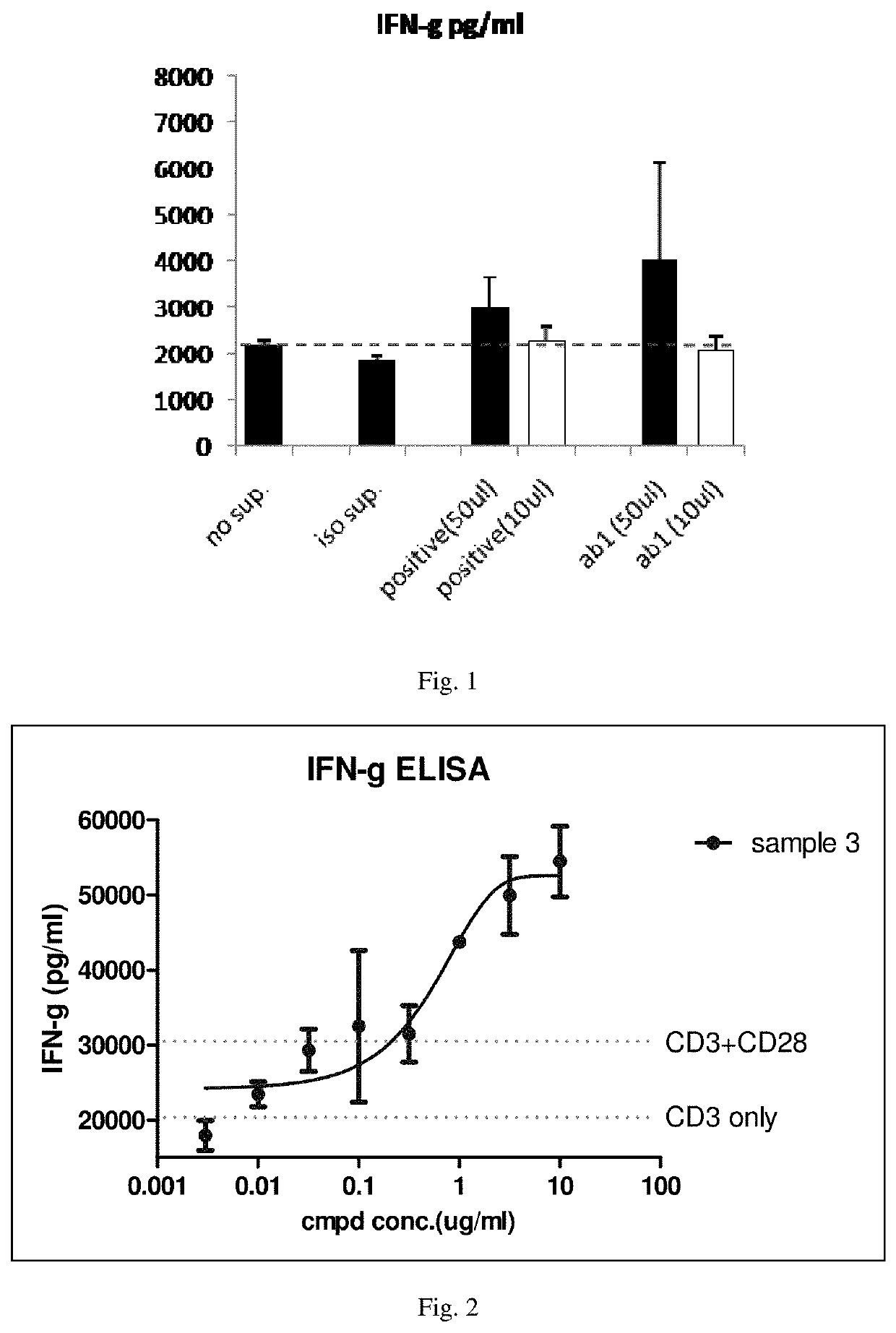
Patents
Literature
67 results about "Cdr3 region" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
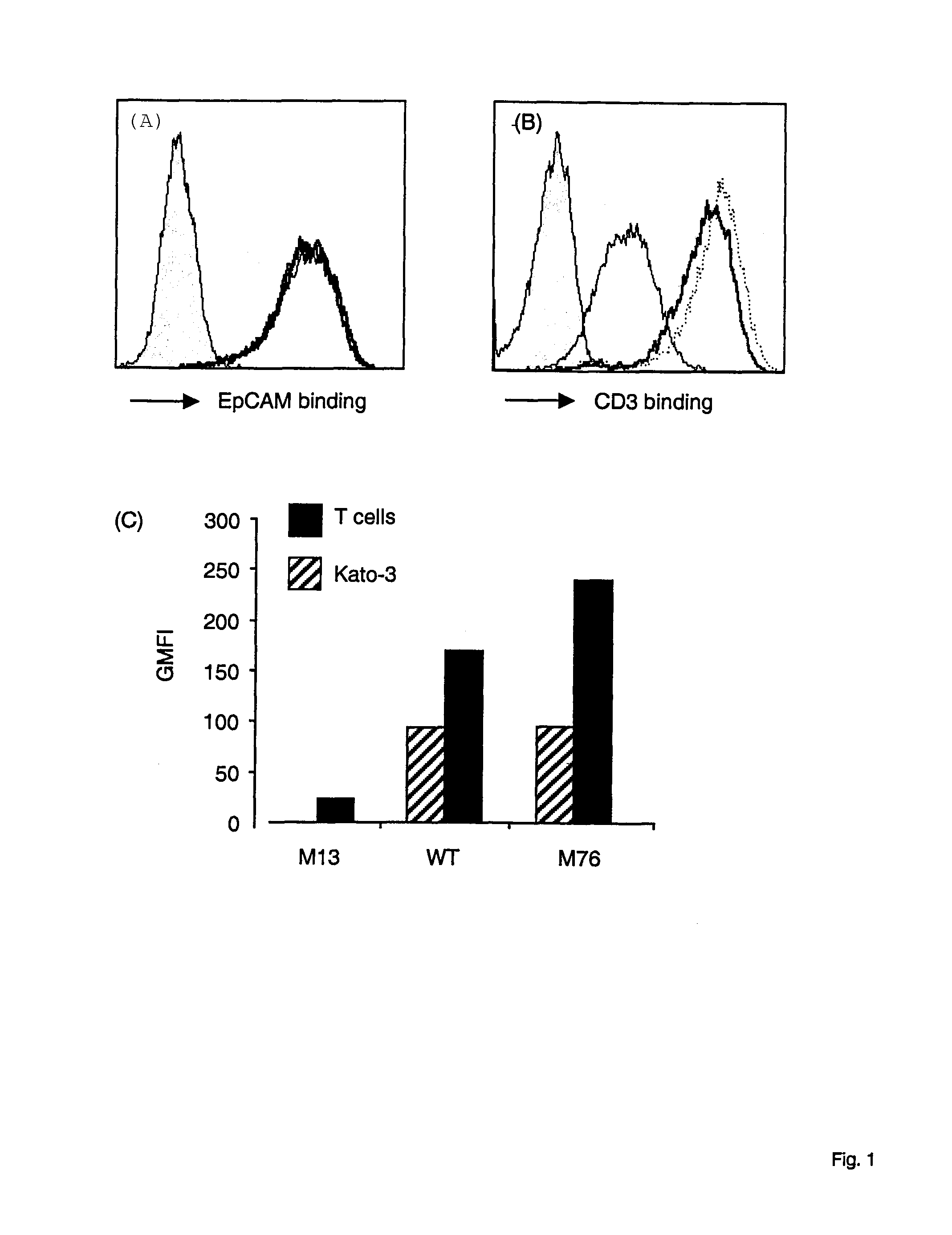
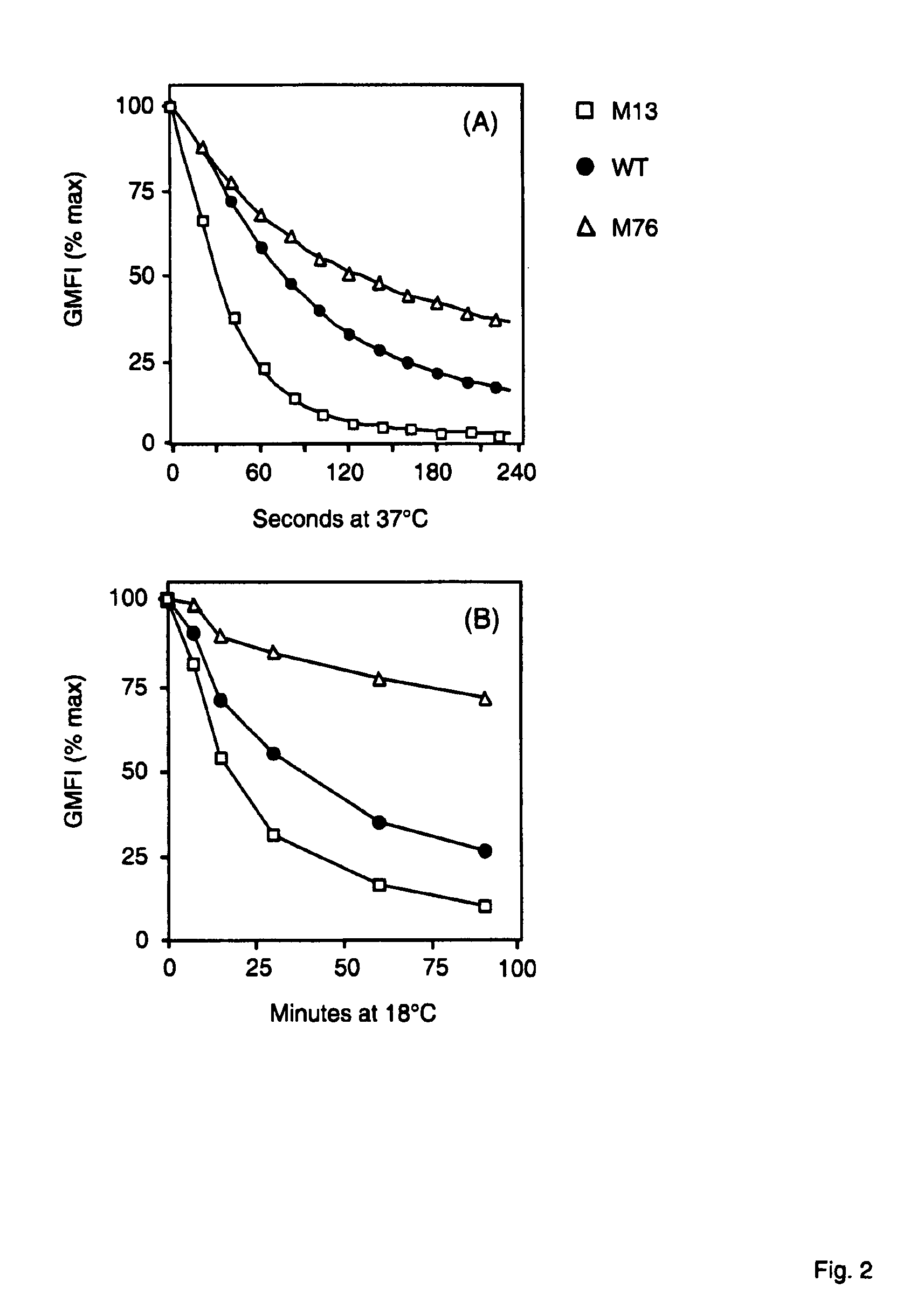
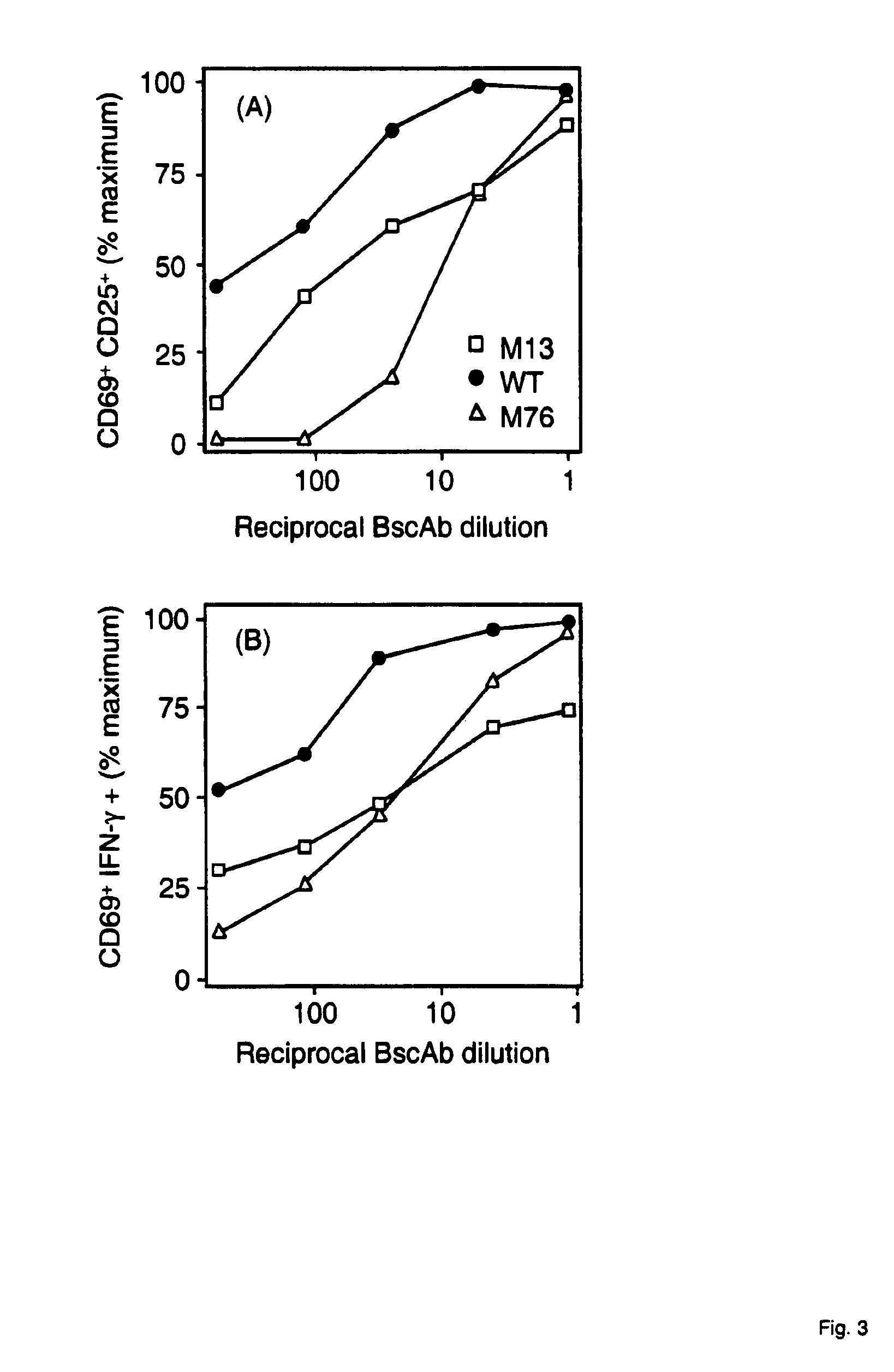
Potent T cell modulating molecules
ActiveUS7820166B2Improve the level ofReduce and eliminate tumorSugar derivativesImmunoglobulins against cell receptors/antigens/surface-determinantsT cellBiology
The present invention relates to a polypeptide construct comprising at least one CDR3 region, wherein at least one of the at least CDR3 regions comprises at least one substitution in the amino acid sequence YYDDHY (SEQ ID NO.1) and wherein the at least one substitution comprises: in the first position of SEQ ID NO.1 a substitution from Y to H; in the second position of SEQ ID NO. 1 a substitution from Y to S, from Y to N, from Y to F or from Y to H; in third position of SEQ ID NO. 1 a substitution from D to N or from D to E; in the forth position of SEQ ID NO. 1 a substitution from D to Q, from D to A, from D to V, from D to E or from D to G; in the fifth position of SEQ ID NO. 1 a substitution from H to Q, from H to P, from H to Y, from H to R or from H to N; or in the sixth position a substitution from Y to N.
Owner:MICROMET AG
Cancer antigen-specific t-cell receptor gene, peptide encoded by the gene, and use of them
InactiveUS20100190163A1Bioreactor/fermenter combinationsBiological substance pretreatmentsAntigenNucleotide
Disclosed are: a nucleotide sequence and an amino acid sequence for CDR3 region of T-cell receptor (TCR) gene of WT1-specific cytotoxic T-cell (CTL) for WT1 protein; a method for the detection or treatment of cancer using the nucleotide sequence or the amino acid sequence; and a chip, a primer set, a kit, an apparatus and the like for use in the detection of cancer, each of which comprises the nucleotide sequence or the amino acid sequence.
Owner:INT INST OF CANCER IMMUNOLOGY INC
Live cell biosensors
InactiveUS20060029946A1Bioreactor/fermenter combinationsCompound screeningPhosphorylationBinding domain
The invention provides dyes, biosensors, and methods for using the dyes and biosensors to detect selected target molecules. The biosensors have a binding domain and a dye, or a dye which is attached directly to the target of interest. Binding domains contemplated by the invention include biomolecules or fragments of biomolecules that interact with target molecules of interest and can be specific to a given conformational state or covalent modification of the molecule (e.g. phosphorylation). In one embodiment, the binding domain of a biosensor is a single chain variable fragment (scFv) with a dye of the invention linked to a CDR3 region. The invention also provides environmentally sensitive dyes useful for detecting changes in the binding, conformational change, or posttranslational modification of the selected target.
Owner:THE SCRIPPS RES INST
Humanized antibody or fragment thereof specific for CD45r0
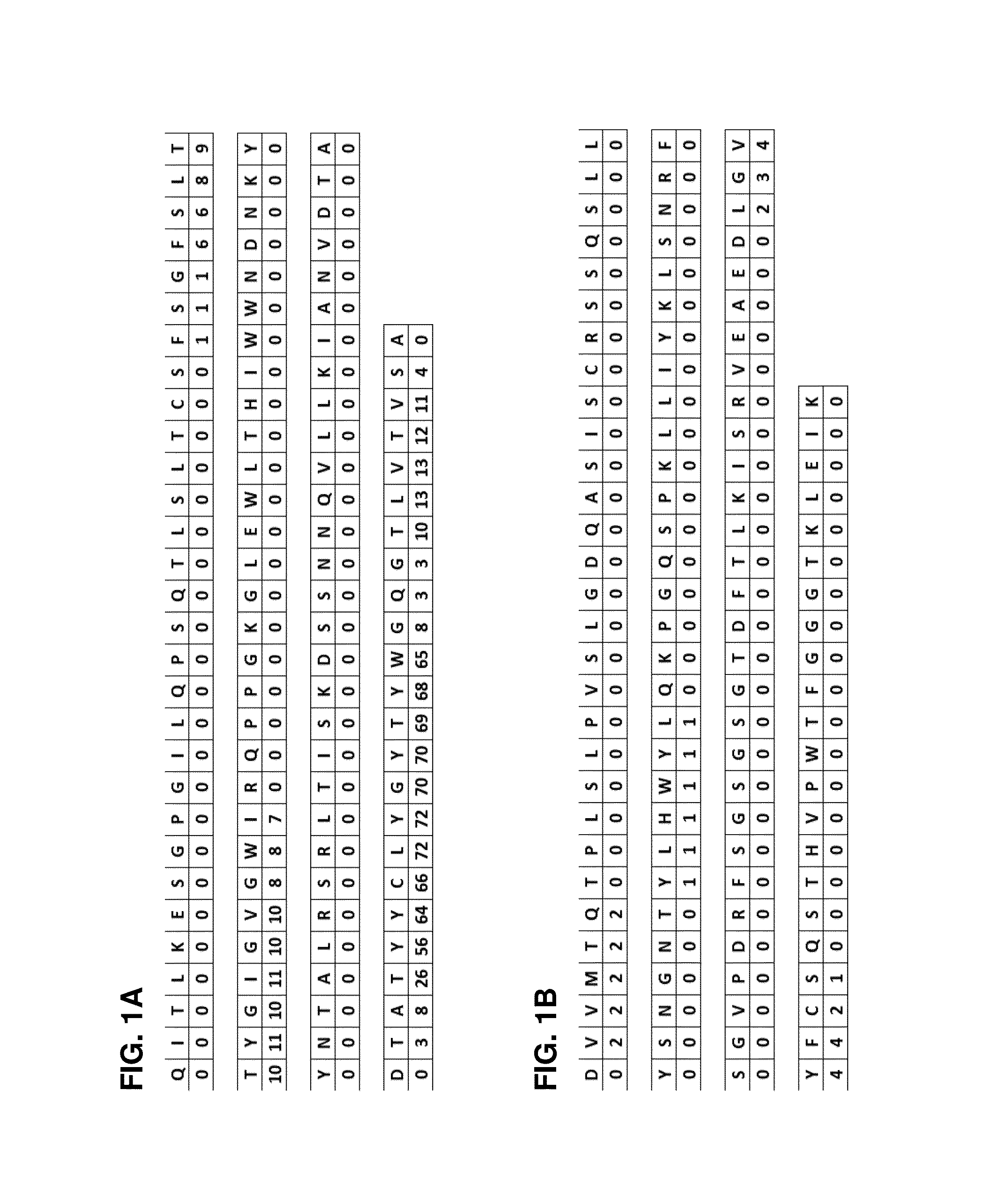
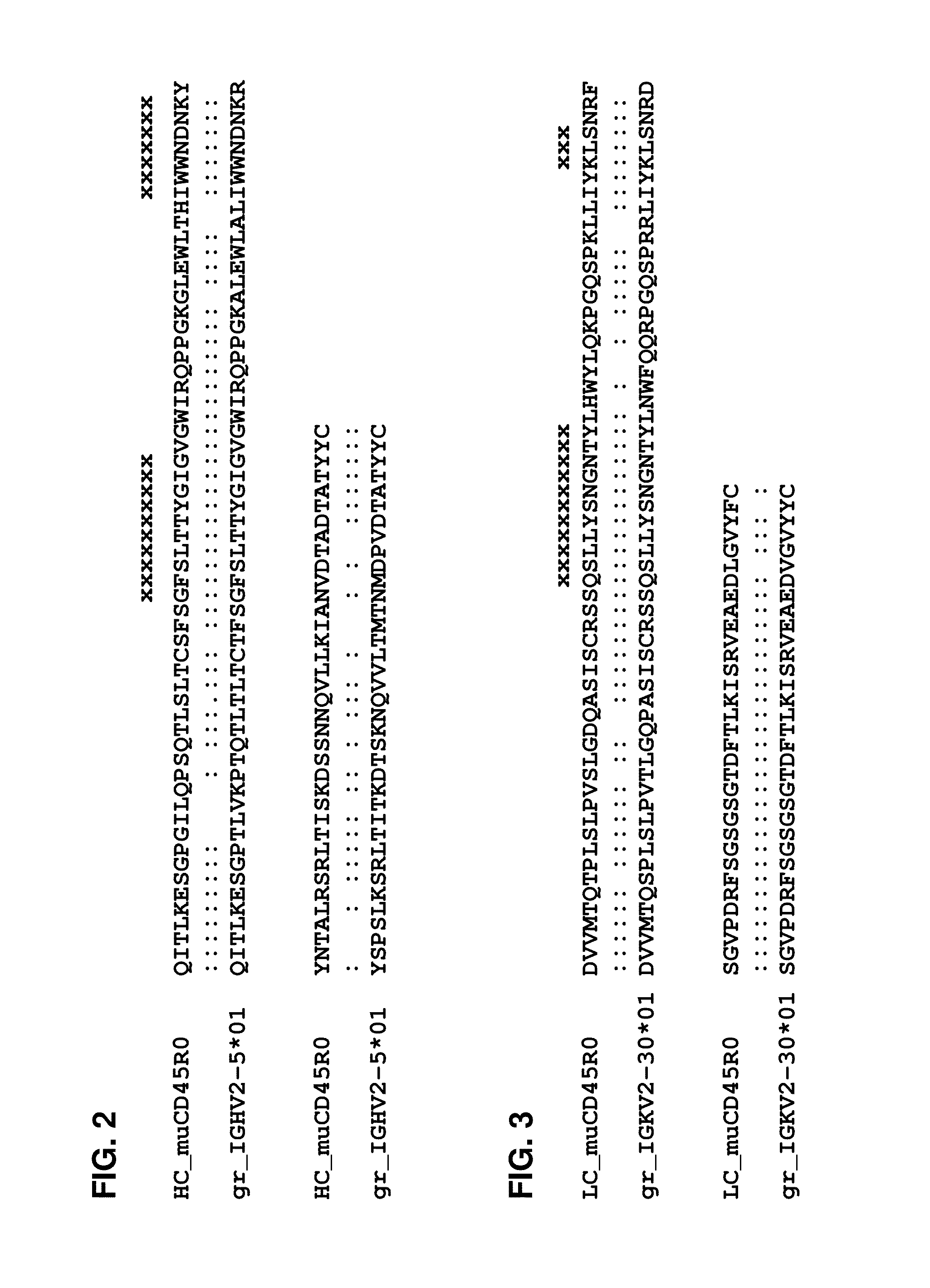
ActiveUS20160152733A1Minimizes immunogenic reactionLower requirementAnimal cellsSugar derivativesAntigenVariable domain
The present invention provides a humanized antibody or fragment thereof specific for the antigen CD45R0, wherein said antibody or fragment thereof comprises a humanized heavy chain variable domain comprising a CDR1 region of SEQ ID NO:1, a CDR2 region of SEQ ID NO:2, and a CDR3 region of SEQ ID NO:3, and a humanized light chain variable domain comprising a CDR1 region of SEQ ID NO:4, a CDR2 region of SEQ ID NO:5, and a CDR3 region of SEQ ID NO:6. It is also provided the use of the present antibody or fragment thereof for enrichment or depletion of CD45R0-expressing cells from a sample comprising CD45R0-expressing cells.
Owner:MILTENYI BIOTEC B V & CO KG
Neutralizing human monoclonal antibodies against hepatitis b virus surface antigen
ActiveUS20160326233A1Efficient and effectiveImmunoglobulins against virusesAntiviralsHeavy chainHepatitis B Surface Antigens
The invention is in the field of medical treatment and prevention. The invention provides an antibody or a part thereof capable of specifically binding to the Hepatitis B surface antigen (HBsAg) and having Hepatitis B Virus (HBV) neutralizing activity, wherein said antibody or fragment thereof comprises a light chain CDR1 region comprising the amino acid sequence of SEQ ID NO: 5, a light chain CDR2 region comprising the amino acid sequence of SEQ ID NO: 6, a light chain CDR3 region comprising the amino acid sequence of SEQ ID NO: 7, a heavy chain CDR1 region comprising the amino acid sequence of SEQ ID NO: 8, a heavy chain CDR2 region comprising the amino acid sequence of SEQ ID NO: 9 and a heavy chain CDR3 region comprising the amino acid sequence of SEQ ID NO: 10.
Owner:MONDELLI MARIO UMBERTO FRANCESCO
Live cell biosensors
Owner:THE SCRIPPS RES INST
Method for simultaneously sequencing multi-sample CDR3 (complementary determining region 3) receptor library with high flux
InactiveCN102443624AHigh-throughput sequencing cost reductionMicrobiological testing/measurementComplementarity determining regionHigh flux
The invention discloses a method for simultaneously sequencing a multi-sample CDR3 (complementary determining region 3) receptor library with a high flux, comprising the following steps of: only designing one set of V-gene family upstream primers in the variable region of a TCR (T cell antigen receptor) or BCR (B cell antigen receptor) chain according to the homology of the V-gene family, then designing C or J gene characteristic downstream primers of the TCR or BCR chain as many as samples and suitable for being paired with the upstream primers, amplifying each sample by one characteristic downstream primer and the same set of upstream primers to obtain a CDR3 region of the TCR or BCR, then mixing all the amplified samples to be a to-be-sequenced sample library, finally sequencing, wherein the characteristic downstream primers are designed by different ATCG basic group compositions. The CDR3 sequences of multiple samples can be simultaneously sequenced by operating one high-flux sequencing program via the method for simultaneously sequencing a multi-sample CDR3 receptor library with a high flux disclosed by the invention, so that the high-flux sequencing cost is greatly decreased. Thus, a high-flux sequencing technology has a wide application value in a sequencing research of a CDR3 receptor library.
Owner:ZUNYI MEDICAL UNIVERSITY
Humanized antibody or fragment thereof specific for CD45R0
ActiveUS9701756B2Minimizes immunogenic reactionLower requirementImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntigenVariable domain
The present invention provides a humanized antibody or fragment thereof specific for the antigen CD45R0, wherein said antibody or fragment thereof comprises a humanized heavy chain variable domain comprising a CDR1 region of SEQ ID NO:1, a CDR2 region of SEQ ID NO:2, and a CDR3 region of SEQ ID NO:3, and a humanized light chain variable domain comprising a CDR1 region of SEQ ID NO:4, a CDR2 region of SEQ ID NO:5, and a CDR3 region of SEQ ID NO:6. It is also provided the use of the present antibody or fragment thereof for enrichment or depletion of CD45R0-expressing cells from a sample comprising CD45R0-expressing cells.
Owner:MILTENYI BIOTEC B V & CO KG
Polypeptides
There is disclosed a polypeptide consisting of between 7 and 100 amino acids and comprising: the sequence of the peptide of any one of SEQ. ID NOS: 1 to 145. There is also disclosed a T-cell receptor, or a peptide-binding fragment thereof, wherein the CDR3 region of the beta chain of the T-cell receptor comprises a glycine residue at position 5 from the N-terminus. The T-cell receptor is capable of binding a peptide consisting of the sequence of SEQ. ID NO. 18, when the peptide is presented on an HLA molecule of a first HLA allele.
Owner:MEDINNOVA AS
Artificial antibody library with super-repertory
ActiveUS8178320B2Improve stabilityProvide stableCompound screeningApoptosis detectionCDNA libraryPcr method
Owner:SHIMIZU NOBUYOSHI +1
Antigenic epitope displaying method based on single domain antibody
ActiveCN102952193AOvercoming the problem of poor immunogenicityAvoid technical complexityPeptide preparation methodsImmunoglobulinsEpitopeSingle-domain antibody
The invention belongs to the technical field of biology and specifically relates to an antigenic epitope displaying method based on a single domain antibody. Specifically, an exogenous antigenic epitope is displayed in a CDR3 region of the single domain antibody, and the single domain antibody skeleton protein which displays the exogenous antigenic epitope on the surface of a molecule can be used for preparing an antibody with a too small epitope, be also used as a substitutive antigen of an exogenous antigen and used for immunizing animals and preparing antibodies aiming at the exogenous antigen. In addition, the antigenic epitope displaying method based on a single domain antibody skeleton can also be used for preparing epitope vaccins.
Owner:BEIJING VICNOVO SCI TECH
Cancer antigen-specific t-cell receptor gene, peptide encoded by the gene, and use of them
Owner:INT INST OF CANCER IMMUNOLOGY INC
CD22 single domain antibody, nucleotide sequence, reagent kit, CAR-T virus vector and CAR-T cell
ActiveCN111217908AHigh activityHigh expressionAntibody mimetics/scaffoldsGenetically modified cellsSingle-domain antibodyT cell
The invention discloses a CD22 single domain antibody, a nucleotide sequence, a reagent kit, a CAR-T virus vector and a CAR-T cell. The amino acid sequences of the CD22 single domain antibody sequentially comprise FR1, CDR1, FR2, CDR2, FR3, CDR3 and FR4 regions, wherein the FR1, FR2, FR3 and FR4 regions are framework region amino acid sequences, the CDR1, CDR2 and CDR3 regions are variable regionamino acid sequences, and the amino acid sequences are shown as table 1. According to the technical scheme, through a method combining genetic engineering, the single domain antibody of specific binding CD22 protein is obtained. The antibody specificity combination CD22 protein is high in specific binding capacity and high in affinity.
Owner:SHENZHEN PREGENE BIOPHARMA CO LTD
HPV6L1 protein-resisting antibody, preparation method therefor and application thereof
ActiveCN105153302ANo cross reactionStrong specificityImmunoglobulins against virusesTissue cultureHeavy chainCdr3 region
The invention provides an antibody or an antigen bound part which is specifically bound to a human papilloma virus low-risk sub type-type 6 L1 protein and / or VLP. The antibody or the antigen bound part comprises CDR1, CDR2 and CDR3 regions of a heavy chain variable region shown in SEQ ID NO. 1-3 and CDR1, CDR2 and CDR3 regions of a light chain variable region shown in SEQ ID NO. 4-6. The antibody or the antigen bound part which is specifically bound with the human papilloma virus low-risk sub type-type 6 L1 protein and / or VLP has no cross reactions with most of other HPV sub types such as HPV11, 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68 and the like, is high in specificity, has a characteristic of neutralizing a HPV6 pseudovirus and blocking infection of the pseudovirus, and is high in affinity. A twin-antibody sandwiched ELISA detection kit established by two antibodies can be used for detecting the presence or level of HPV6L1 protein and the antibody can be further used for treatment of patients and passive immunity of susceptible population and has a good application prospect.
Owner:NAT VACCINE & SERUM INST
Human Neutralizing Anti-Kit Antibodies and Uses Thereof
InactiveUS20160264680A1Avoid signalingReduced viabilityAntibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsHeavy chainBiology
The invention relates to human neutralizing anti-KIT antibodies and uses thereof. More particularly, the invention relates to an antibody comprising a heavy chain variable region comprising SEQ ID NO: 2 in the H-CDR1 region, SEQ ID NO: 3 in the H-CDR2 region and SEQ ID NO: 4 in the H-CDR3 region; and a light chain variable region comprising SEQ ID NO: 6 in the L-CDR1 region, SEQ ID NO: 7 in the L-CDR2 region and SEQ ID NO: 8 in the L-CDR3 region. The invention also relates to an antibody comprising a heavy chain variable region comprising SEQ ID NO: 10 in the H-CDR1 region, SEQ ID NO: 11 in the H-CDR2 region and SEQ ID NO: 12 in the H-CDR3 region; and a light chain variable region comprising SEQ ID NO: 14 in the L-CDR1 region, SEQ ID NO: 15 in the L-CDR2 region and SEQ ID NO: 16 in the L-CDR3 region.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +5
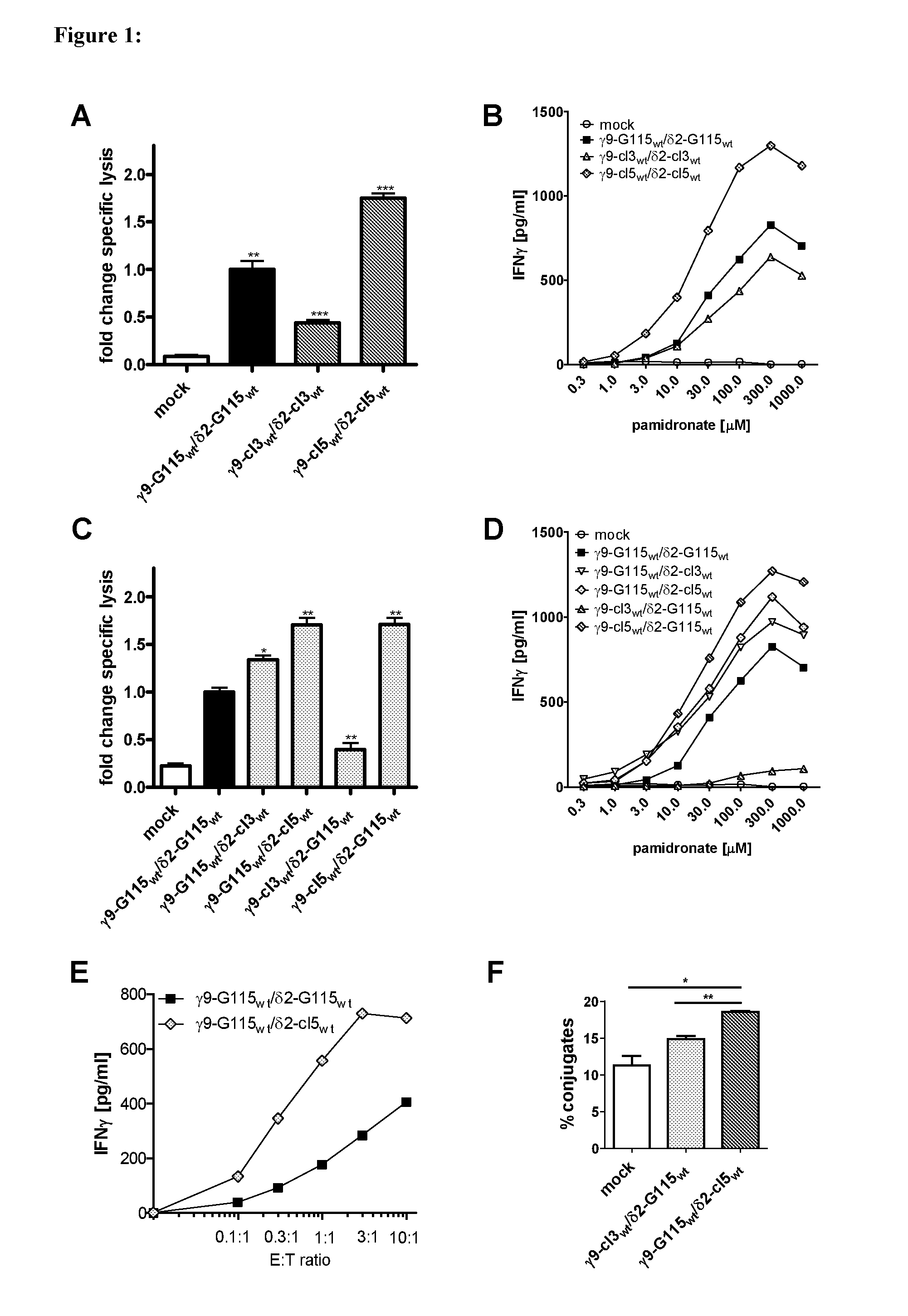
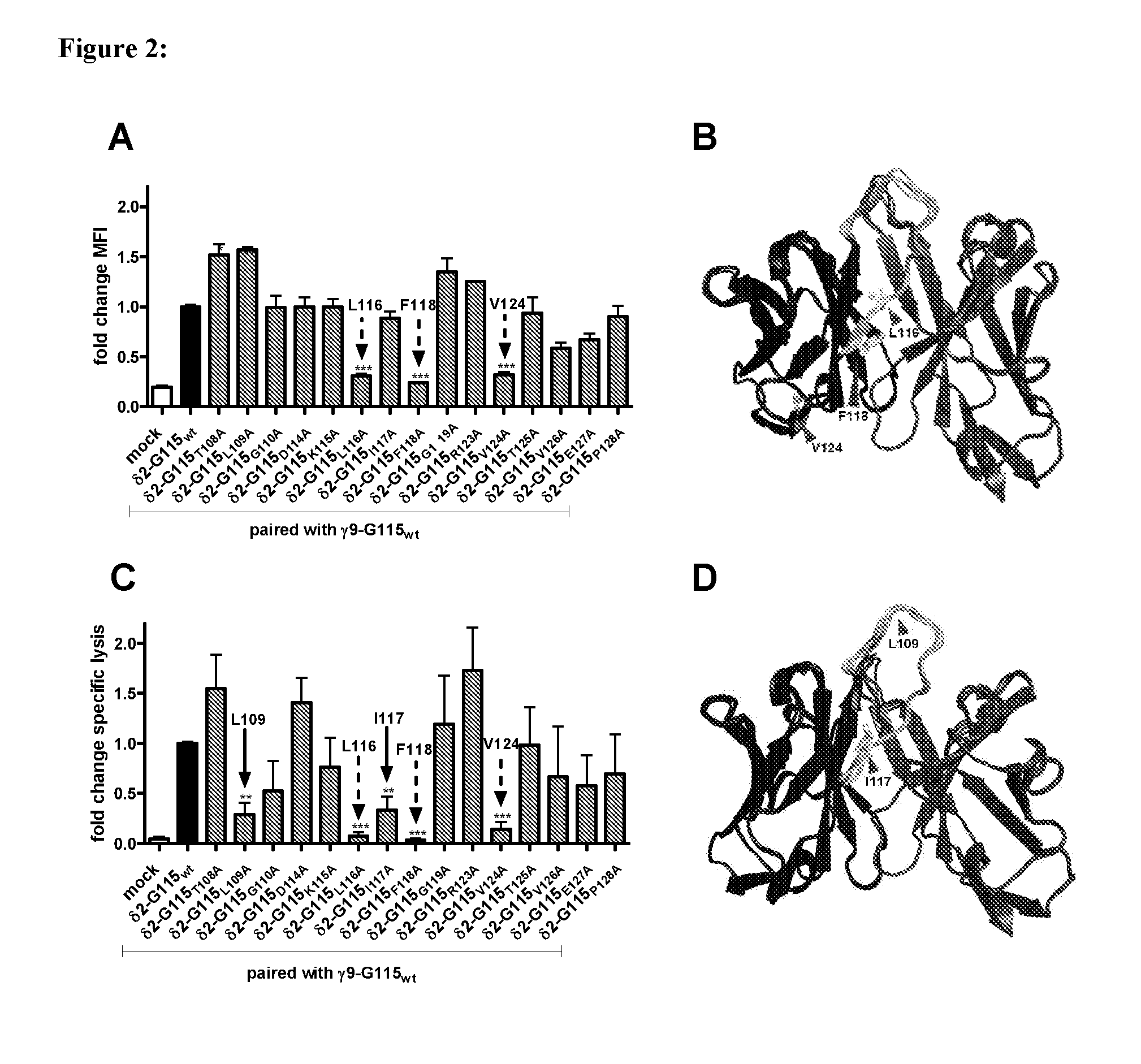
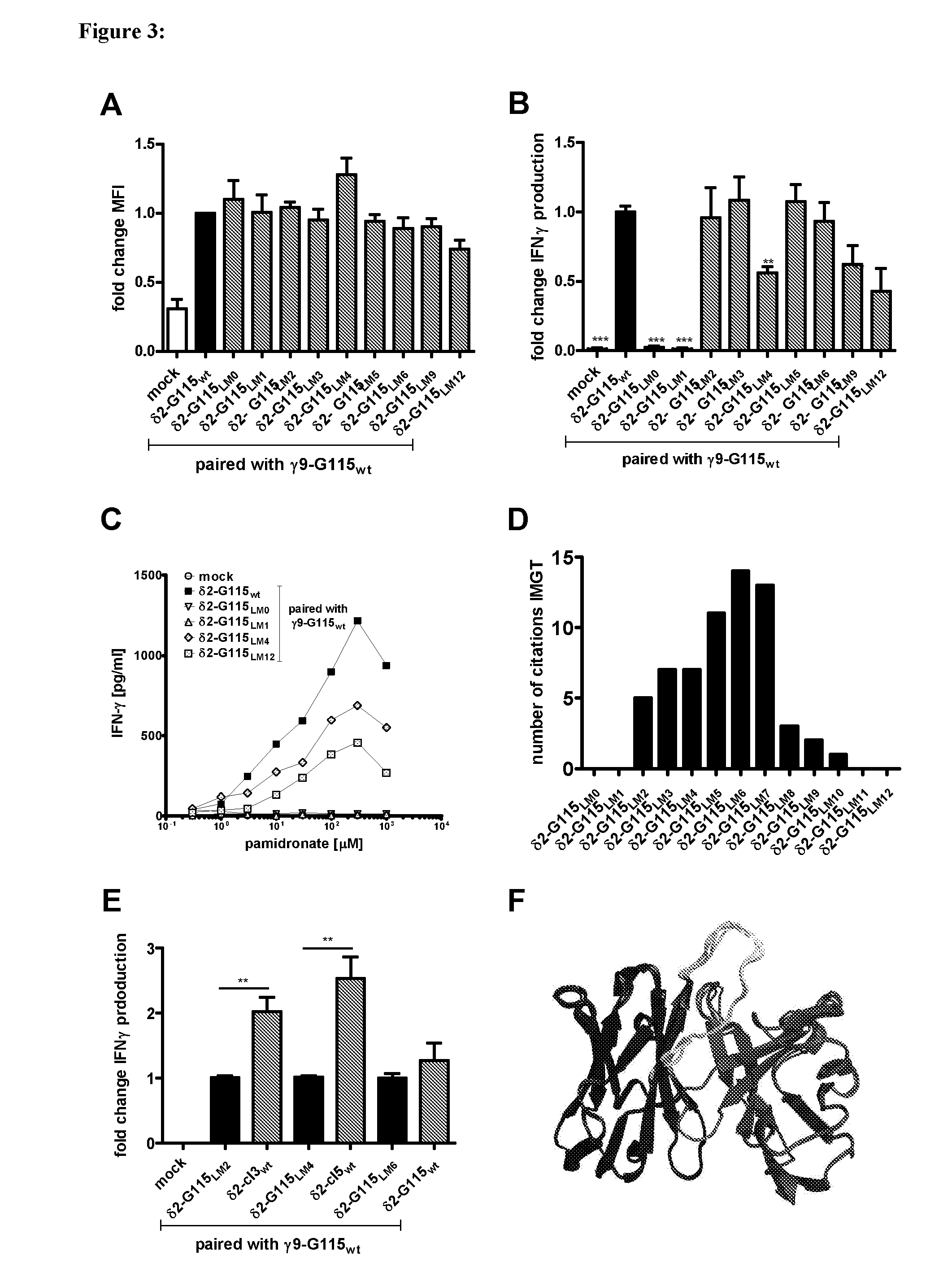
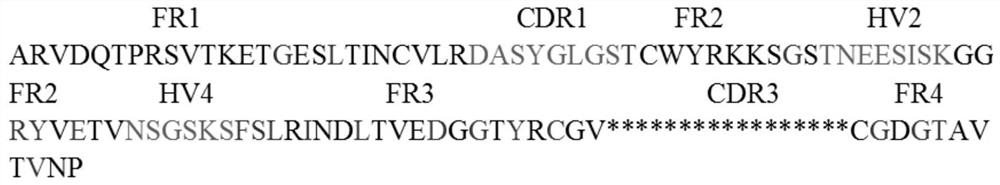
Combinatorial gamma 9 delta 2 t cell receptor chain exchange
The current invention provides methods to identify γ9δ2T-cell receptors (γ9δ2TCR) that mediate anti-tumour responses. Surprisingly, it was now found that the CDR3 regions of the γ9-T-cell receptor chain and the δ2-T-Cell receptor chain (δ2TCR chain) are of importance. Based on these findings, combinatorial-γδTCR-chain-exchange (CTE) is proposed as an efficient method for identifying γ9δ2TCRs that mediate anti-tumour responses. Using the method of the invention, specific sequences of the respective γ9TCR and δ2TCR chains were identified that mediate anti-tumour responses. Hence, the invention further provides for specific γ9δ2TCRs, or fragments thereof, that may be used e.g. in diagnostics or treatment of cancer. The invention further provides for nucleic acid sequences, genetic constructs and retroviral vectors that can be used to express the γ9δ2TCRs according to the invention.
Owner:GADETA BV
Anti-PD-L1 nano antibody and application thereof
ActiveCN111909272AImprove stabilityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAntiendomysial antibodiesPharmaceutical drug
The invention provides an anti-PD-L1 nano antibody and application thereof. The sequence of the antibody comprises an FR region, a CDR region and an HV region, wherein the FR region comprises an FR1 region with an amino acid sequence as shown in SEQ ID NO.1, an FR2 region with an amino acid sequence as shown in SEQ ID NO.2, an FR3 region with an amino acid sequence as shown in SEQ ID NO.3 and an FR4 region with an amino acid sequence as shown in SEQ ID NO.4; the CDR region comprises a CDR1 region with an amino acid sequence shown as SEQ ID NO.5 and a CDR3 region with an amino acid sequence shown as any one of SEQ ID NO.8 to SEQ ID NO.12; and the HV region comprises an HV2 region with an amino acid sequence as shown in SEQ ID NO.6 and an HV4 region with an amino acid sequence as shown in SEQ ID NO.7. The anti-PD-L1 nano antibody screened on the basis of the novel shark V-NAR framework sequence has excellent stability and can provide a new variety for research, development and acquisition of novel antitumor drugs.
Owner:EAST CHINA UNIV OF SCI & TECH
Antibody specially bound with Norovirus GII.4 genotype VP1 protein and/or VLP and preparation method and application of antibody
ActiveCN109265542AStrong specificityHigh affinityBiological material analysisImmunoglobulins against virusesHeavy chainAntigen binding
The invention discloses an antibody specially bound with Norovirus GII.4 genotype VP1 protein and / or VLP. The antibody comprises CDR1, CDR2 and CDR3 regions of a heavy chain variable region shown in SEQ ID NO. 1-3 and / or The CDR1, CDR2 and CDR3 regions of the light chain variable region shown in SEQ ID NO. 4-6. The antibody or antigen binding part of Norovirus GII.4 genotype VP1 protein and / or VLPprovided by the present invention, has no cross-reaction with GI.1 genotype, is high in specificity, has the characteristic of blocking binding between Norovirus GII.4 genotype VP1 protein and / or VLPand HBGA, is high in affinity, can be used for detecting the presence or level of Norovirus GII.4 genotype VP1 protein and / or VLP in a sample developing kits, as well as treating patients and performing passive immunization on susceptible populations, and has good application prospect.
Owner:NAT VACCINE & SERUM INST
Anti-CD123 antibody and application thereof
ActiveCN112851807AHigh affinityStrong binding specificityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunologic disordersAutoimmune condition
The invention discloses an antibody specifically binding to CD123. The antibody comprises: a) CDR1, CDR2 and CDR3 regions of a heavy-chain variable region shown as SEQ ID No. 1 to 3 or regions 80% or more homogeneous with the CDR1, CDR2 and CDR3 regions; or b) CDR1, CDR2 and CDR3 regions of a heavy-chain variable region shown as SEQ ID No. 4 to 6 or regions 80% or more homogeneous with the CDR1, CDR2 and CDR3 regions; or c) CDR1, CDR2 and CDR3 regions of a heavy-chain variable region shown as SEQ ID No. 7 to 9 or regions 80% or more homogeneous with the CDR1, CDR2 and CDR3 regions. The monoclonal antibody can be used for detecting cells expressing CD123, and can also be applied to tumor immunotherapy independently or be applied to tumor immunotherapy in combination with other methods; that is, the monoclonal antibody can be effectively applied to preparation of drugs for treating tumors, infectious diseases and autoimmune diseases, resisting immunological rejection and the like.
Owner:INST OF HEMATOLOGY & BLOOD DISEASES HOSPITAL CHINESE ACADEMY OF MEDICAL SCI & PEKING UNION MEDICAL COLLEGE +1
Method for simultaneously sequencing multi-sample CDR3 (complementary determining region 3) receptor library with high flux
InactiveCN102443624BHigh-throughput sequencing cost reductionMicrobiological testing/measurementComplementarity determining regionHigh flux
The invention discloses a method for simultaneously sequencing a multi-sample CDR3 (complementary determining region 3) receptor library with a high flux, comprising the following steps of: only designing one set of V-gene family upstream primers in the variable region of a TCR (T cell antigen receptor) or BCR (B cell antigen receptor) chain according to the homology of the V-gene family, then designing C or J gene characteristic downstream primers of the TCR or BCR chain as many as samples and suitable for being paired with the upstream primers, amplifying each sample by one characteristic downstream primer and the same set of upstream primers to obtain a CDR3 region of the TCR or BCR, then mixing all the amplified samples to be a to-be-sequenced sample library, finally sequencing, wherein the characteristic downstream primers are designed by different ATCG basic group compositions. The CDR3 sequences of multiple samples can be simultaneously sequenced by operating one high-flux sequencing program via the method for simultaneously sequencing a multi-sample CDR3 receptor library with a high flux disclosed by the invention, so that the high-flux sequencing cost is greatly decreased. Thus, a high-flux sequencing technology has a wide application value in a sequencing research of a CDR3 receptor library.
Owner:ZUNYI MEDICAL UNIVERSITY
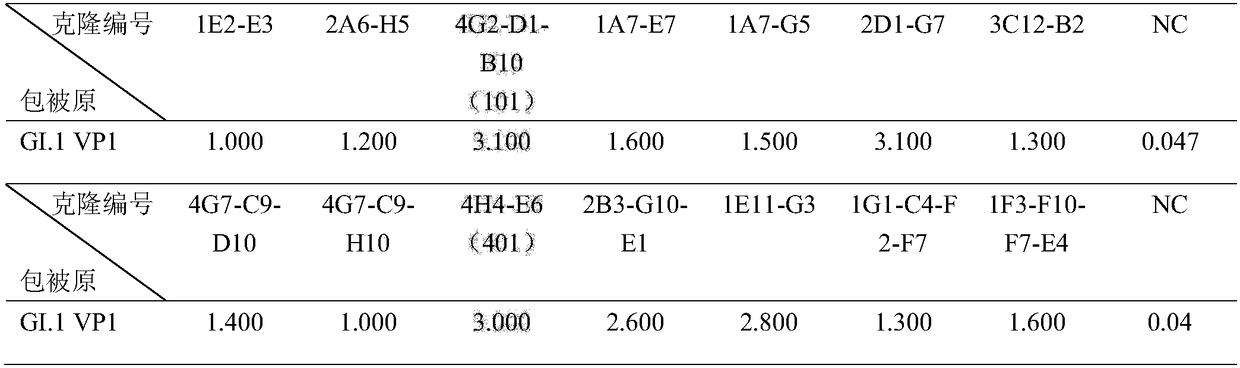
Antibody capable of realizing specific binding with norovirus GI.1 gene type VP1 protein and/or VLP, and preparation method and applications thereof
ActiveCN109180810AStrong specificityHigh affinityBiological material analysisImmunoglobulins against virusesHeavy chainNorovirus GI
The invention discloses an antibody capable of realizing specific binding with norovirus GI.1 gene type VP1 protein and / or VLP, and a preparation method and applications thereof. The antibody containsCDR1, CDR2 and CDR3 zones containing heavy chain variable regions represented by SEQ ID NO.1-3, and / or CDR1, CDR2 and CDR3 zones containing light chain variable regions represented by SEQ ID NO.4-6.No cross reaction of the norovirus GI.1 gene type VP1 protein and / or VLP antibody or an antigen binding part with GII.4 gene type is caused; the specificity is high; the antibody is capable of blocking binding of norovirus GI.1 gene type VP1 protein and / or VLP with HBGA; the endophilicity is high; the antibody can be used for research and development of kits used for detecting existing or contentof norovirus GI.1 gene type VP1 protein and / or VLP in samples, and treatment on patients and passive immunity on susceptible population, and is promising in application prospect.
Owner:NAT VACCINE & SERUM INST +1
Artificial antibody library with super-repertory
ActiveUS20060057632A1Quality improvementEasy to introduceCompound screeningPeptide librariesCDNA libraryPcr method
An artificial antibody library with a super-repertory (1011 or more) is constructed by: using a cDNA library as a template, amplifying a fragment containing the CDR1 and CDR2s regions of the VH or VL region of immunoglobulin gene and a fragment containing the CDR3 region each by the PCR method; integrating the VH library and the VL library, which are little contaiminated with unexpressionable repertory and have high safety, into an nono-expression vector; transferring it into a host; and then shuffling the VH region in the VH library with the VL region in the VL library.
Owner:SHIMIZU NOBUYOSHI +1
Method for constructing mouse TCR alpha CDR3 region library
PendingCN107829145AAccurate detectionMicrobiological testing/measurementLibrary creationTotal rnaMouse tissue
The invention relates to a method for constructing a mouse TCR alpha CDR3 region sequencing library. The method mainly comprises the following steps: (1) extracting total RNA from mouse tissues or whole blood; (2) reversely transcribing the RNA into cDNA, ligating a linker sequence to the end of the long-stranded cDNA, and amplifying TCR alpha containing a CDR3 region through a universal C-terminal primer and a linker sequence primer; (3) fragmenting the TCR alpha through Tn5 transposase, and enriching a CDR3 region sequence; and (4) carrying out sequencing by an illumina high-throughput sequencing platform. The method is suitable for library construction of tissues containing various types of T cells, and body fluids, can highly-efficiently amplify the mouse TCR alpha through a 5'RACE technology in an agonic manner, and solves the problems of difficulty in control of the template copy number and the offset of the product, caused by multiplex PCR amplification; and the Tn5 enzyme's ability to simultaneously complete DNA fragmentation and linker ligation and a specific primer are used to fast and accurately enrich and construct a library of the hypervariable region (CDR3 region) ofthe TCR alpha, so pertinent sequencing, easy data analysis and sequencing cost saving are achieved.
Owner:重庆天科雅生物科技有限公司
Method for constructing human TCR alpha CDR3 region library
The invention relates to a method for constructing a human TCR alpha CDR3 region sequencing library. The method mainly comprises the following steps: (1) extracting total RNA from human tissues or whole blood; (2) reversely transcribing the RNA into cDNA, ligating a linker sequence to the end of the long-stranded cDNA, and amplifying TCR alpha containing a CDR3 region through a universal C-terminal primer and a linker sequence primer; (3) fragmenting the TCR alpha through Tn5 transposase, and enriching a CDR3 region sequence; and (4) carrying out sequencing by an illumina high-throughput sequencing platform. The method is suitable for library construction of tissues and body fluids, containing various types of T cells, can highly-efficiently amplify the human TCR alpha through a 5'RACE technology in an agonic manner, and solves the problems of difficulty in control of the template copy number and the offset of the product, caused by multiplex PCR amplification; and the Tn5 enzyme's ability to simultaneously complete DNA fragmentation and linker ligation and a specific primer are used to fast and accurately enrich and construct a library of the hypervariable region (CDR3 region) ofthe TCR alpha, so pertinent sequencing, easy data analysis and sequencing cost saving are achieved.
Owner:重庆天科雅生物科技有限公司
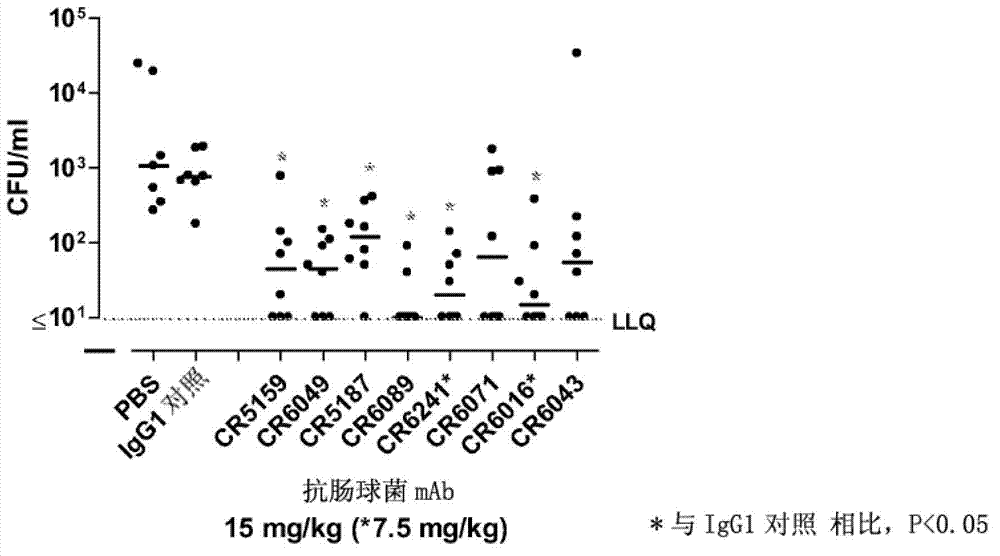
Human binding molecules having killing activity against enterococci and staphylococcus aureus and uses thereof
ActiveCN102731651AGrowth inhibitory activityAntibacterial agentsFungiStaphylococcus cohniiHeavy chain
Disclosed is a human monoclonal antibody, wherein said antibody comprises: a heavy chain CDR1 region comprising amino acid sequence SEQ ID NO: 296; a heavy chain CDR2 region comprising amino acid sequence SEQ ID NO: 297; a heavy chain CDR3 region comprising amino acid sequence SEQ ID NO: 298; a light chain CDR1 region comprising amino acid sequence SEQ ID NO: 299; a light chain CDR2 region comprising amino acid sequence SEQ ID NO: 300; a light chain CDR3 region comprising amino acid sequence SEQ ID NO: 301; characterized in that said antibody has opsonic phagocytic killing activity against at least one strain of each of at least two different Enterococcus species and against at least one strain of Staphylococcus aureus.
Owner:JANSSEN VACCINES & PREVENTION BV
CD123 single domain antibody, nucleic acid sequence, expression vector and kit
ActiveCN110804096AHigh activityHigh expressionBiological material analysisImmunoglobulins against cell receptors/antigens/surface-determinantsSingle-domain antibodyNucleic acid sequencing
The invention discloses a CD123 single domain antibody, a nucleic acid sequence, an expression vector and a kit. An amino acid sequence of the CD123 single domain antibody includes an FR1 region, a CDR1 region, an FR2 region, a CDR2 region, an FR3 region, a CDR3 region and an FR4 region which are formed in sequence, wherein the FR1 region, the FR2 region, the FR3 region and the FR4 region are composed of frame-region amino acid sequences, the CDR1 region, the CDR2 region and the CDR3 region are composed of variable-region amino acid sequences, and the amino acid sequences of the FR1 region, the CDR1 region, the FR2 region, the CDR2 region, the FR3 region, the CDR3 region and the FR4 region are shown in a table 1. According to the technical scheme, in combination with a genetic engineeringmethod, the single domain antibody capable of specifically binding to a CD123 protein is obtained, and the single domain antibody has high specific binding capability of specifically binding to the CD123 protein and high affinity.
Owner:SHENZHEN PREGENE BIOPHARMA CO LTD
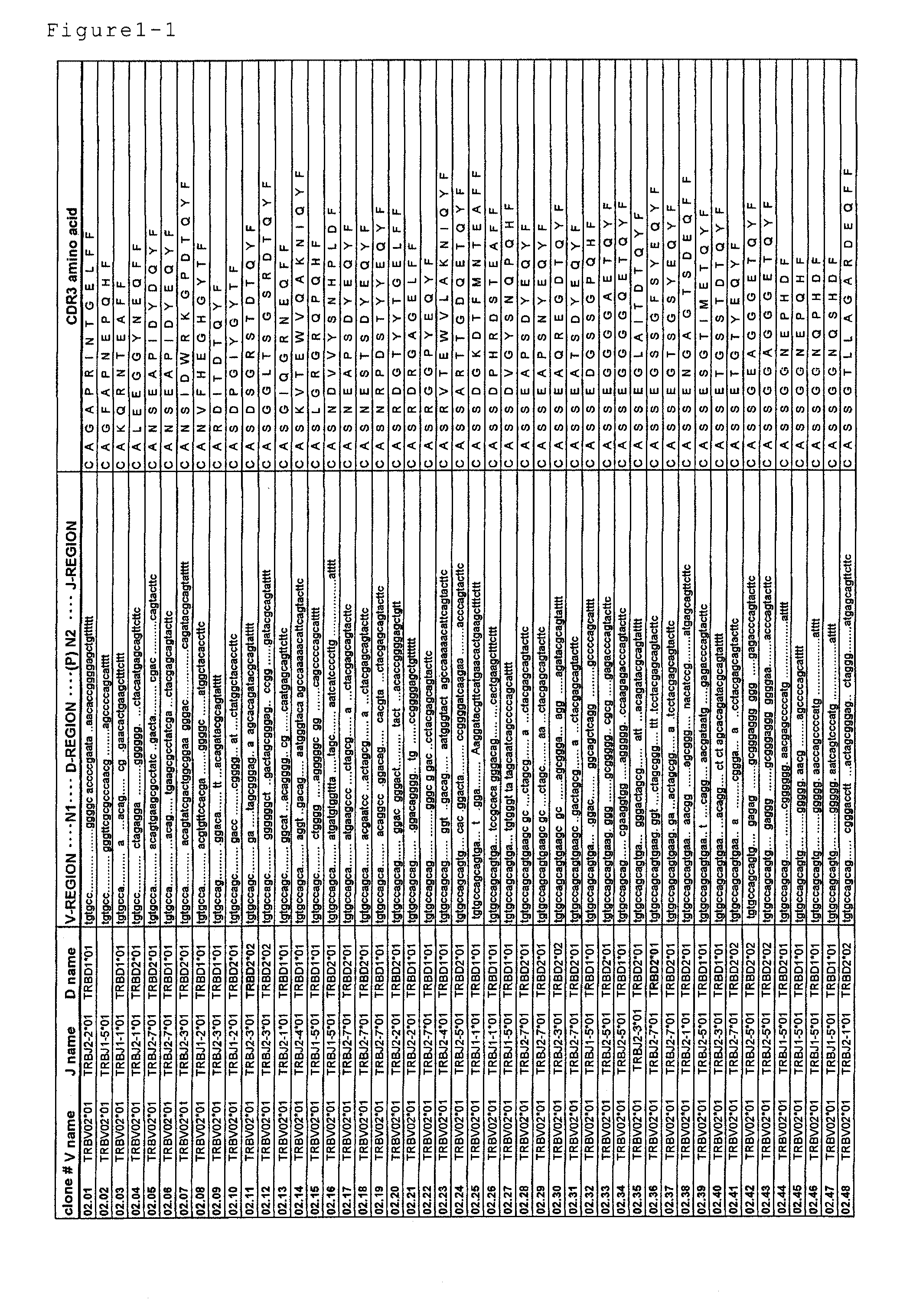
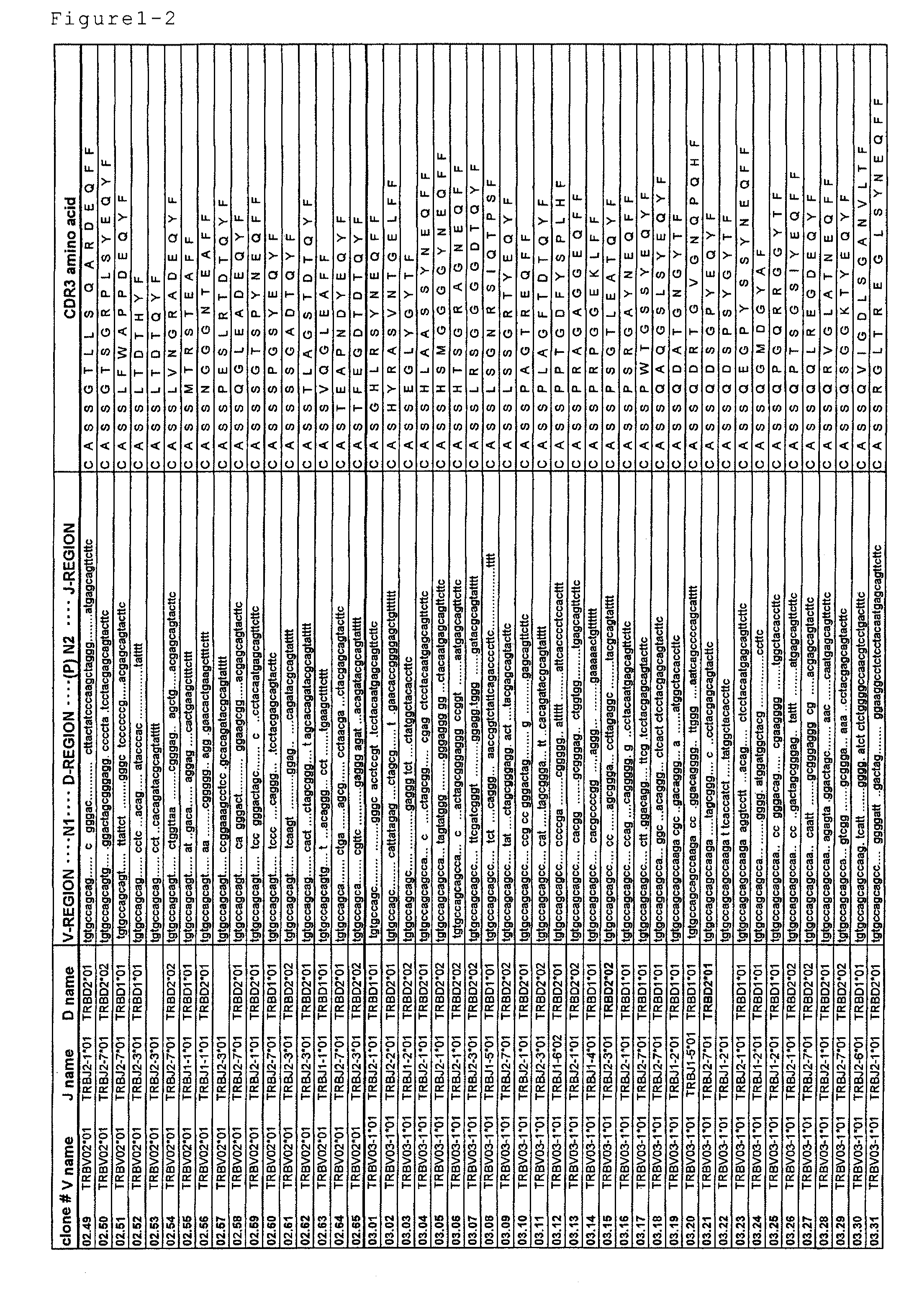
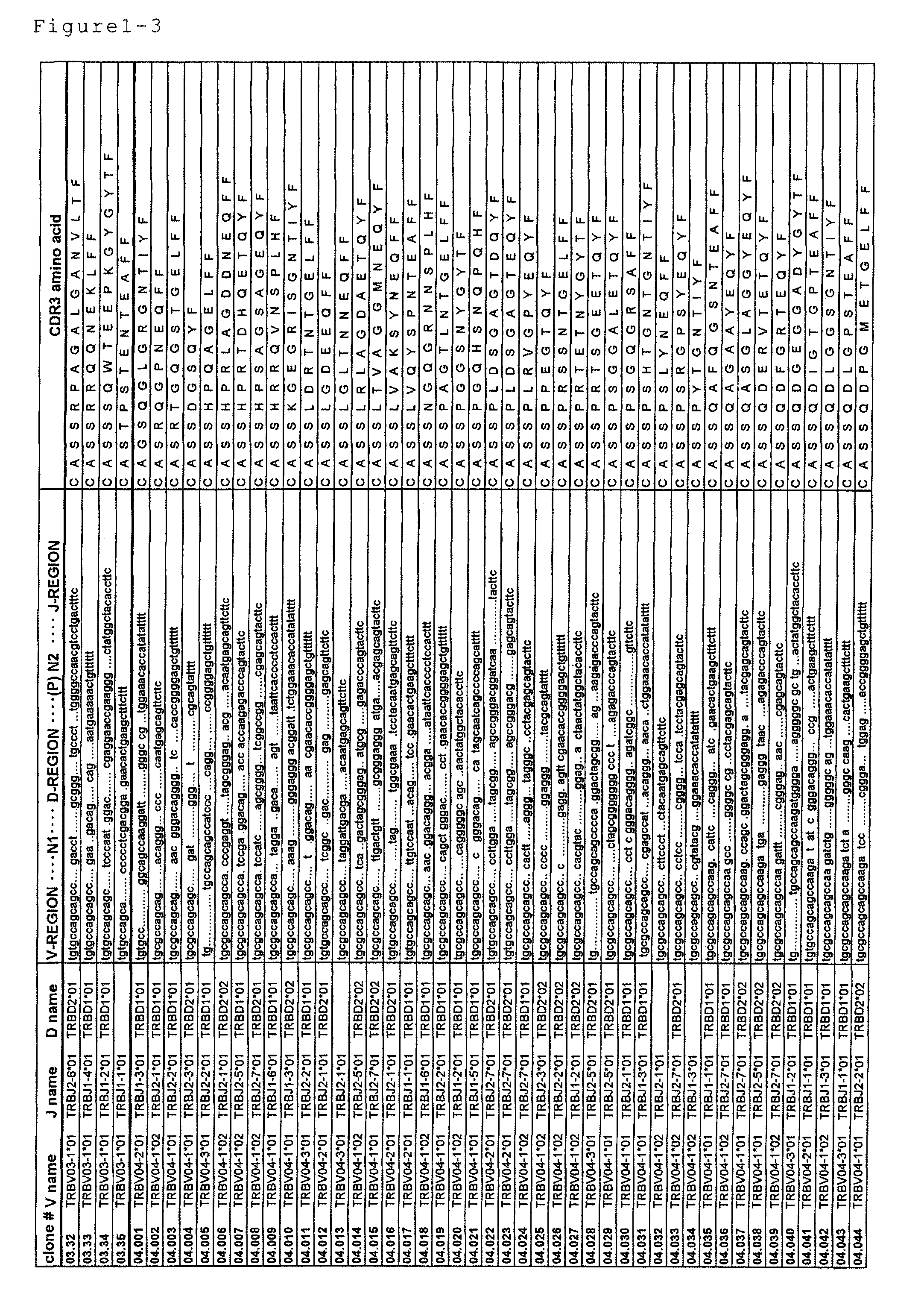
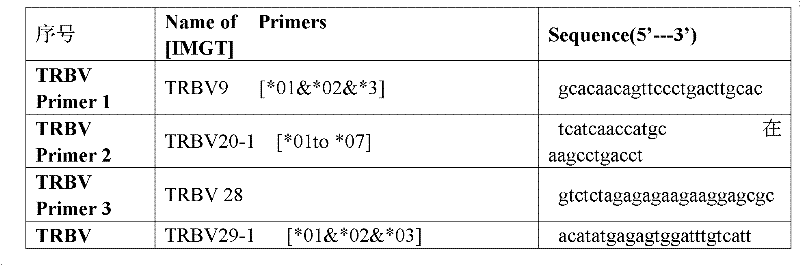
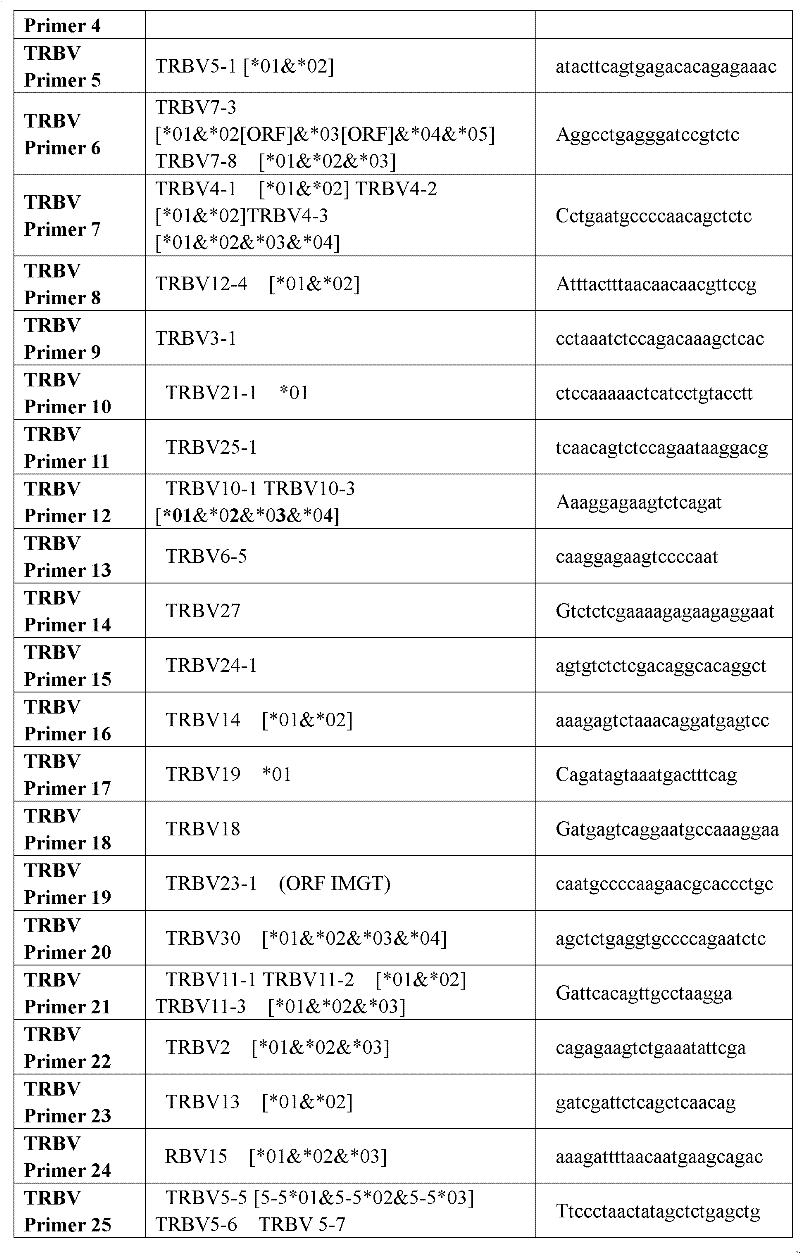
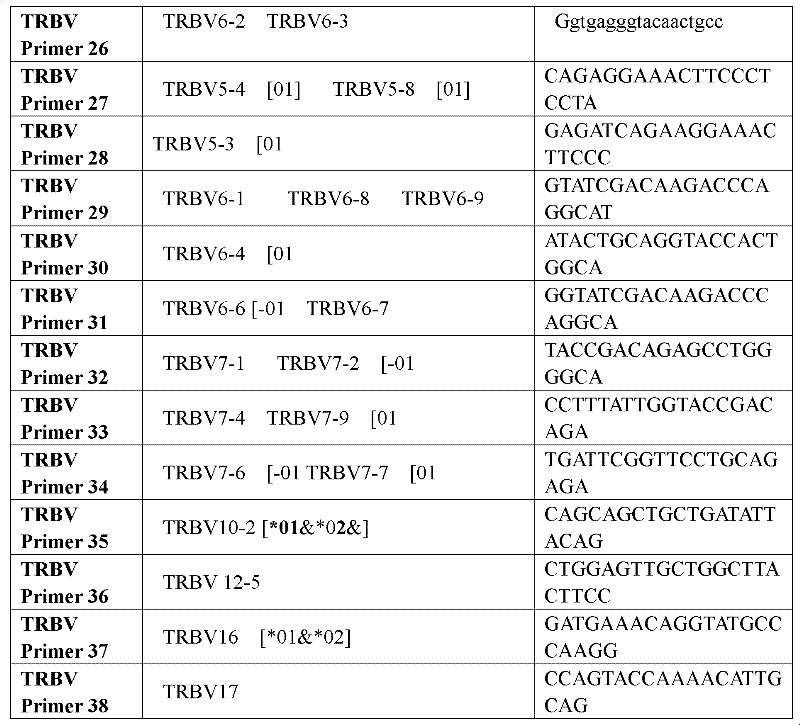
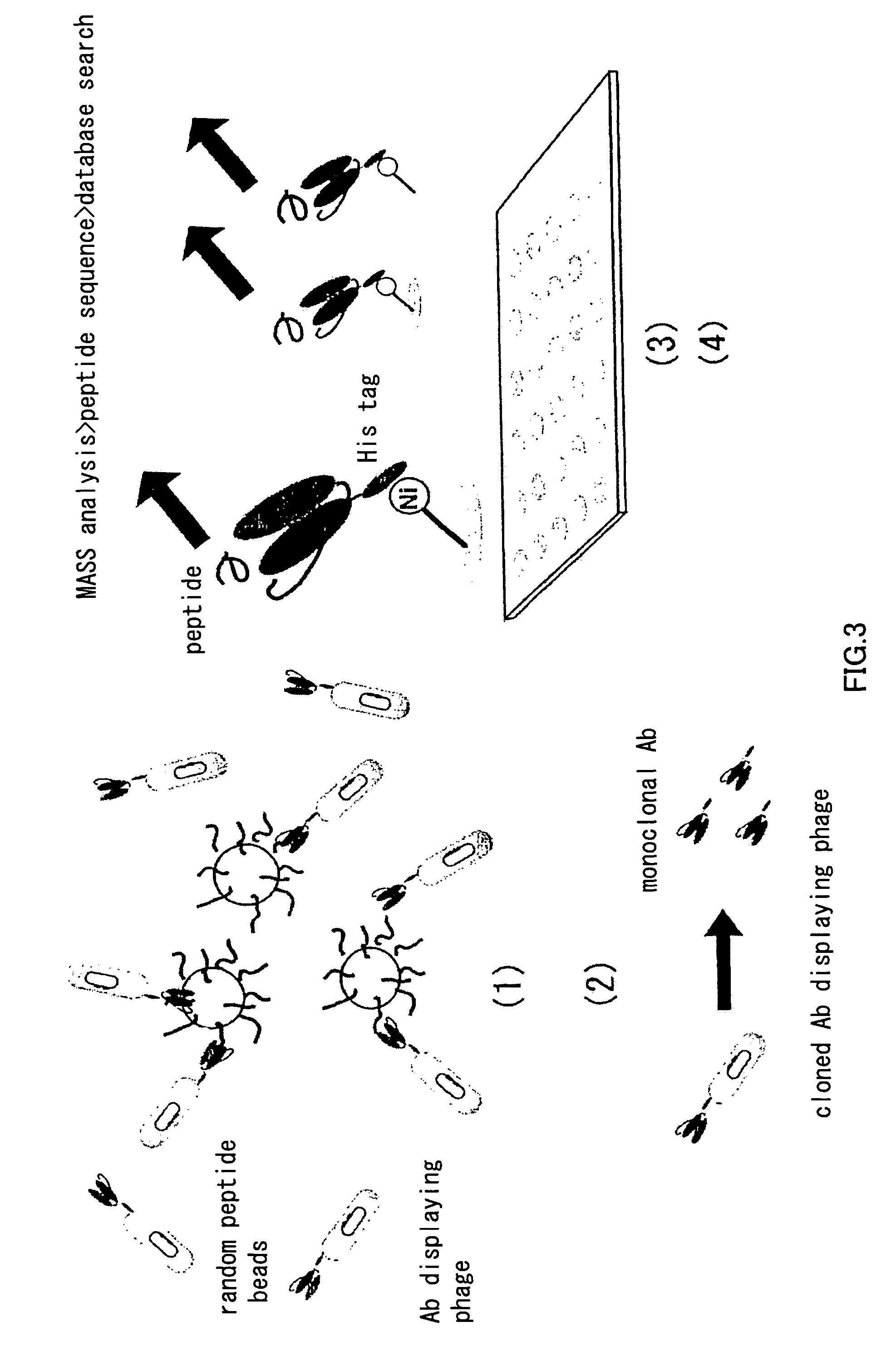
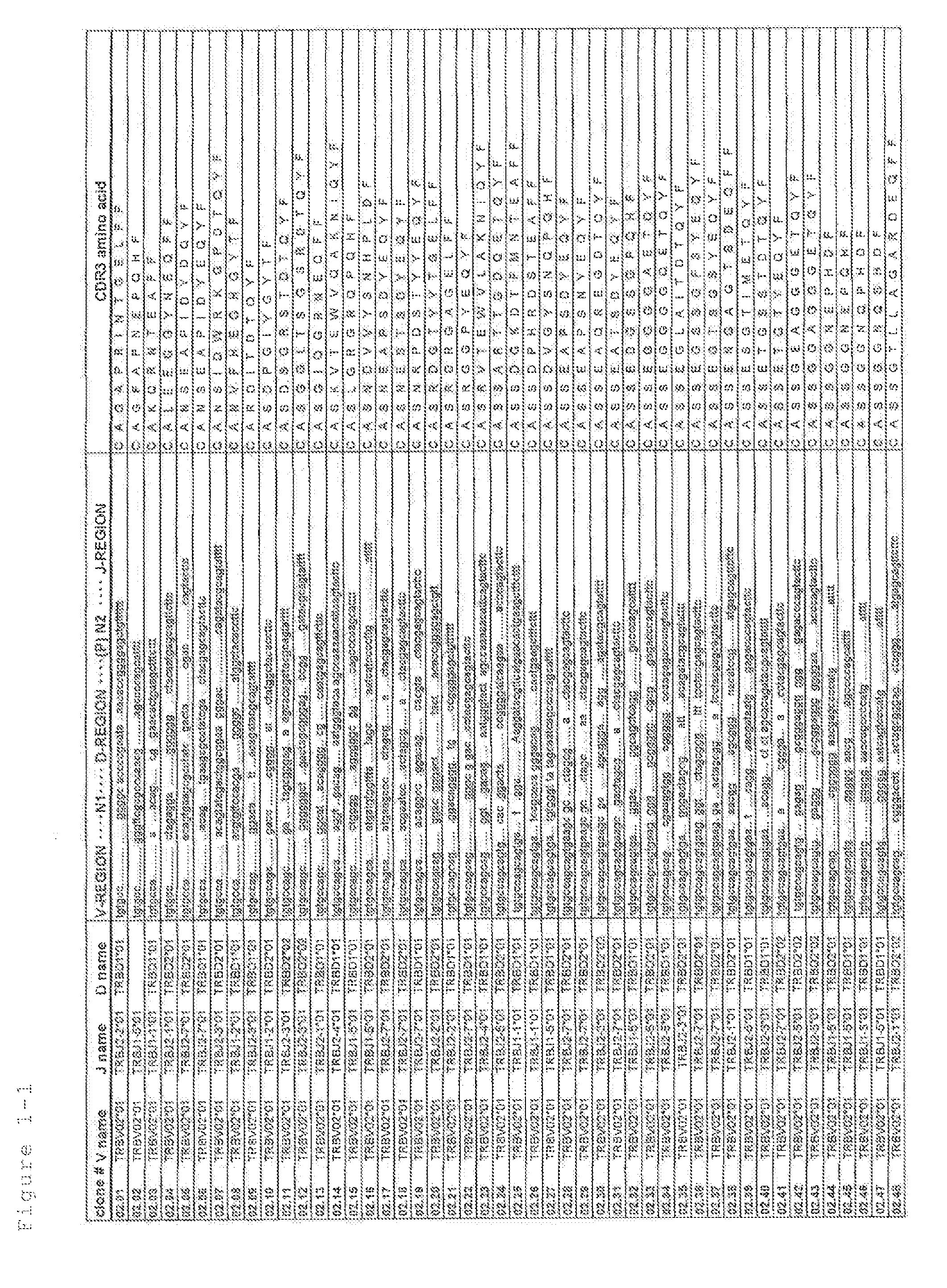
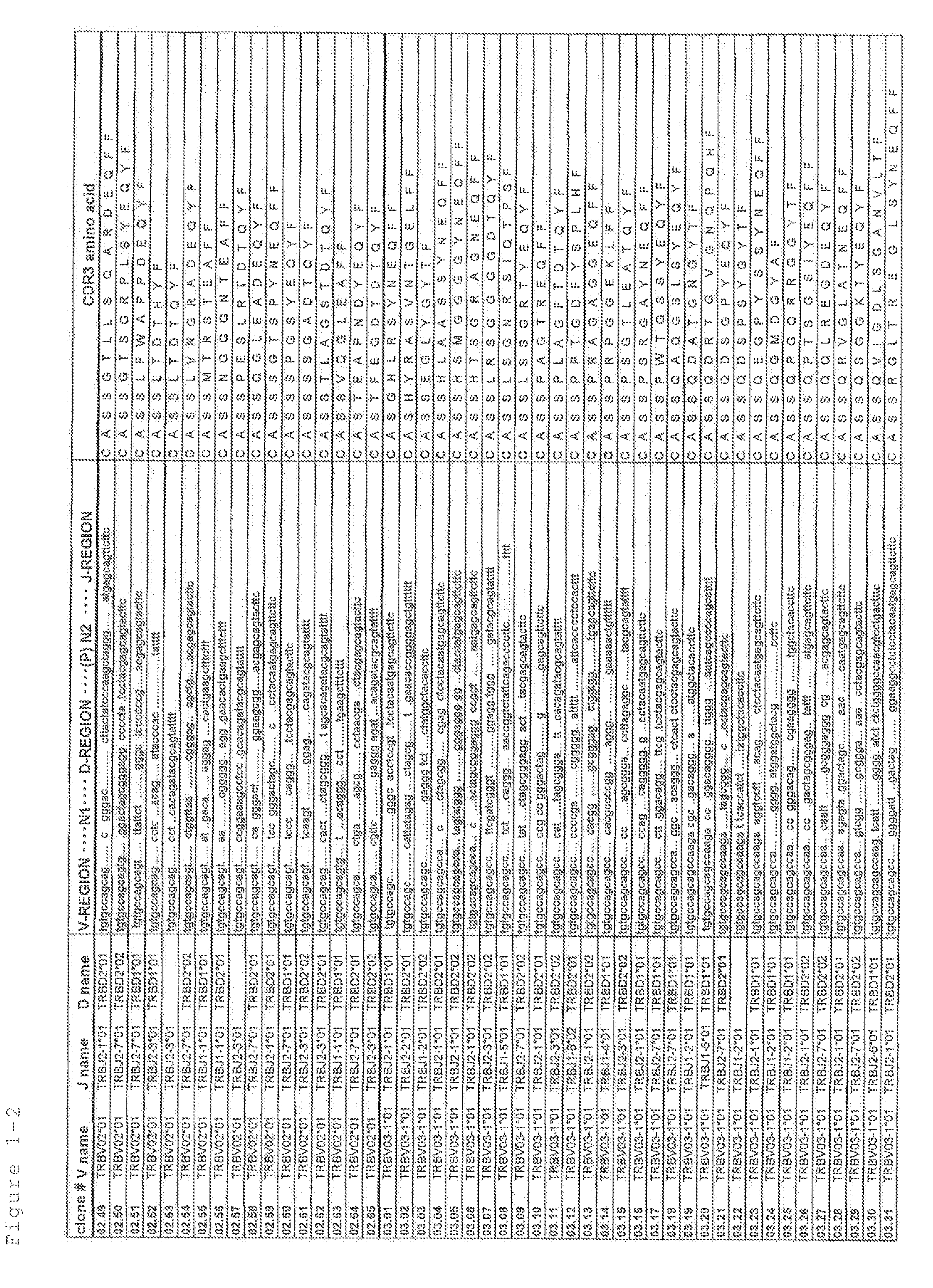
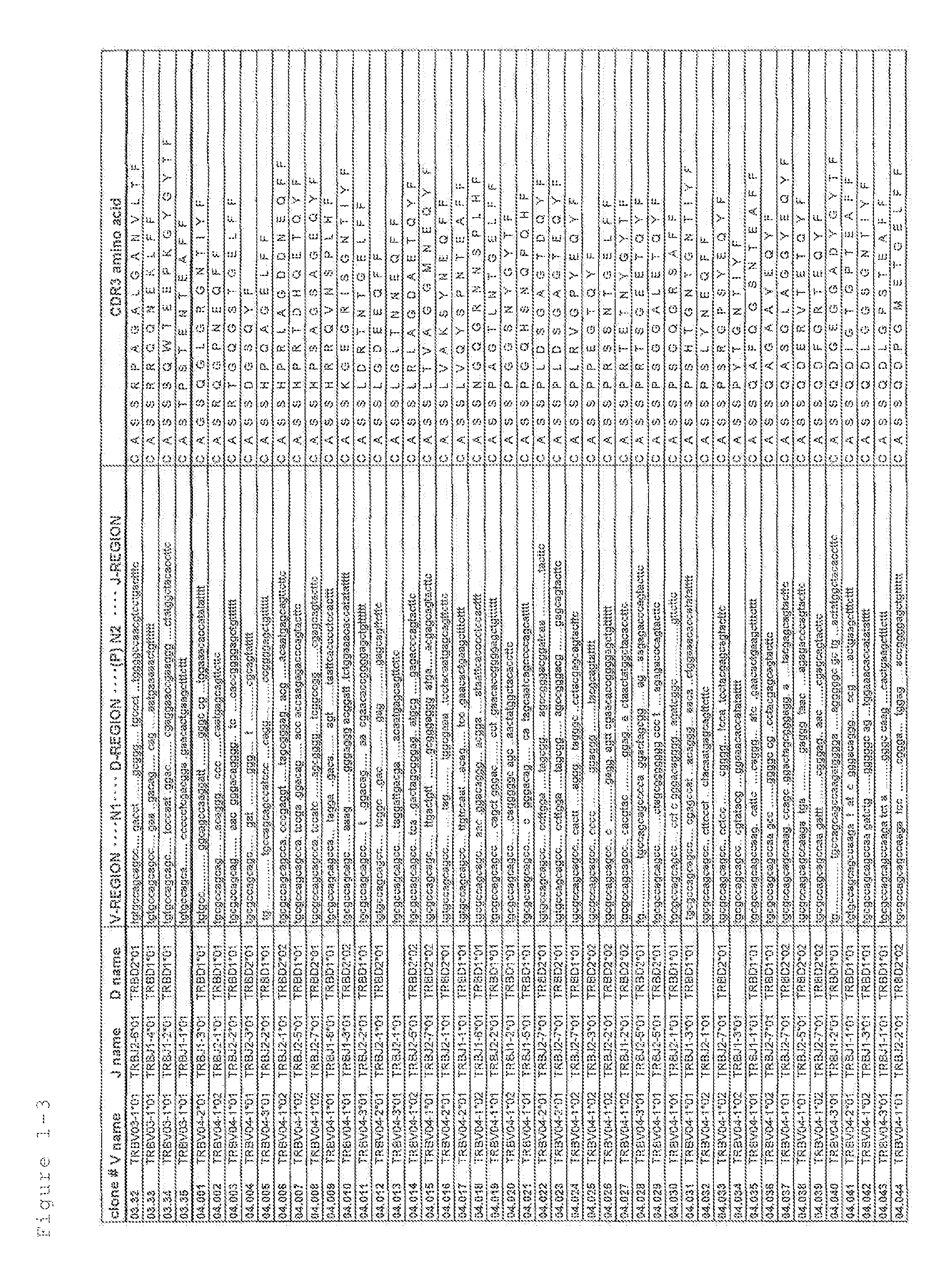
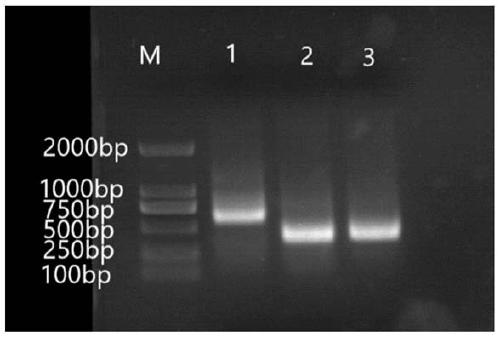
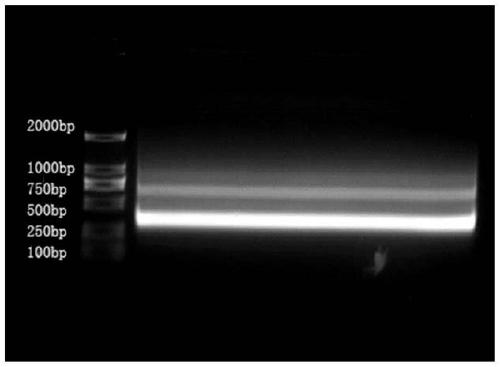
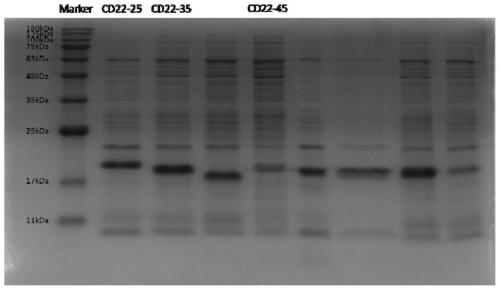
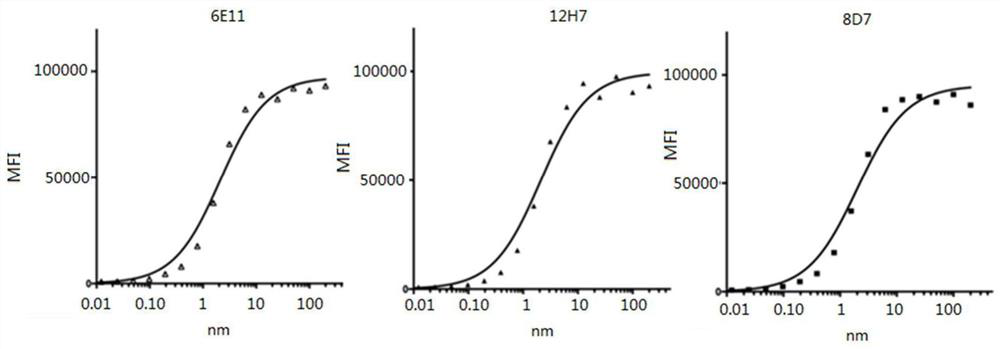
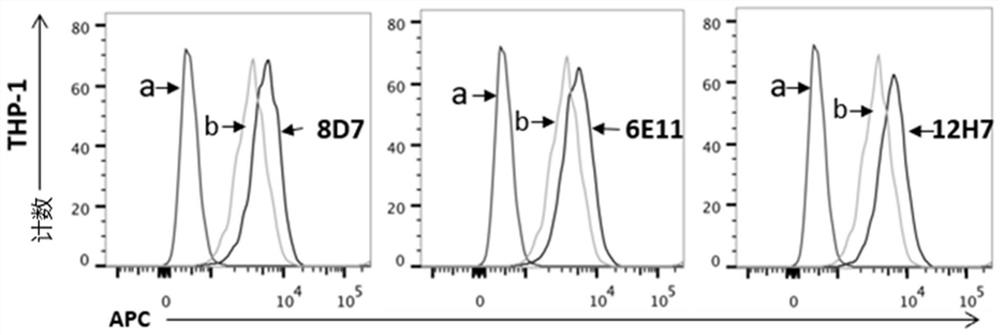
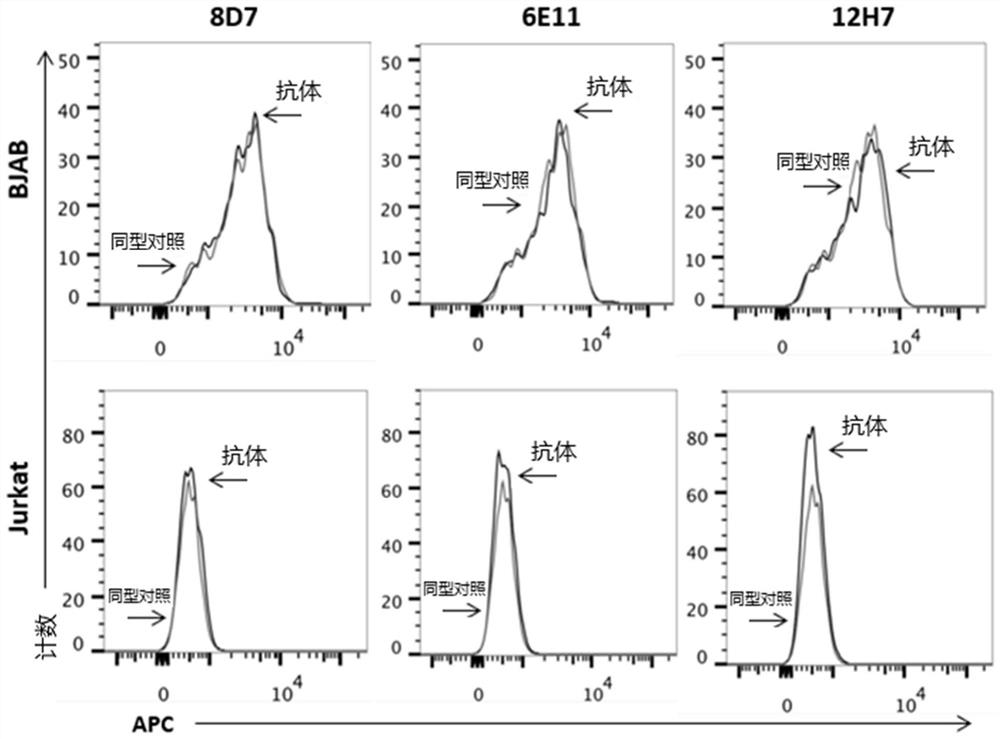
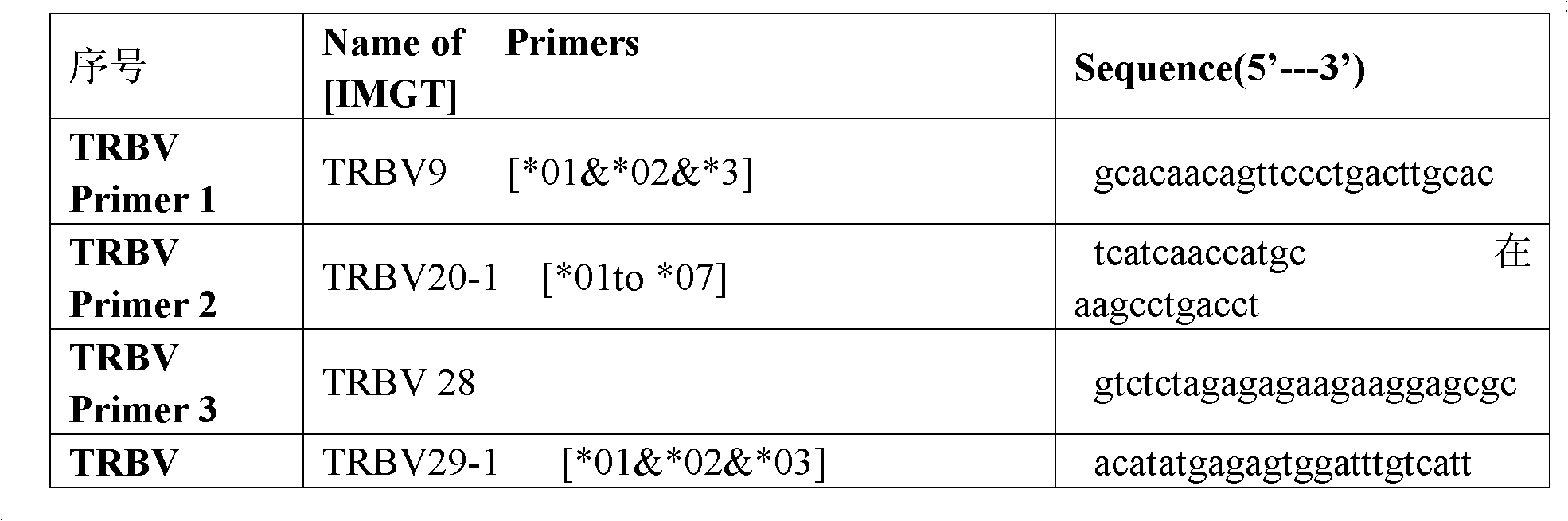
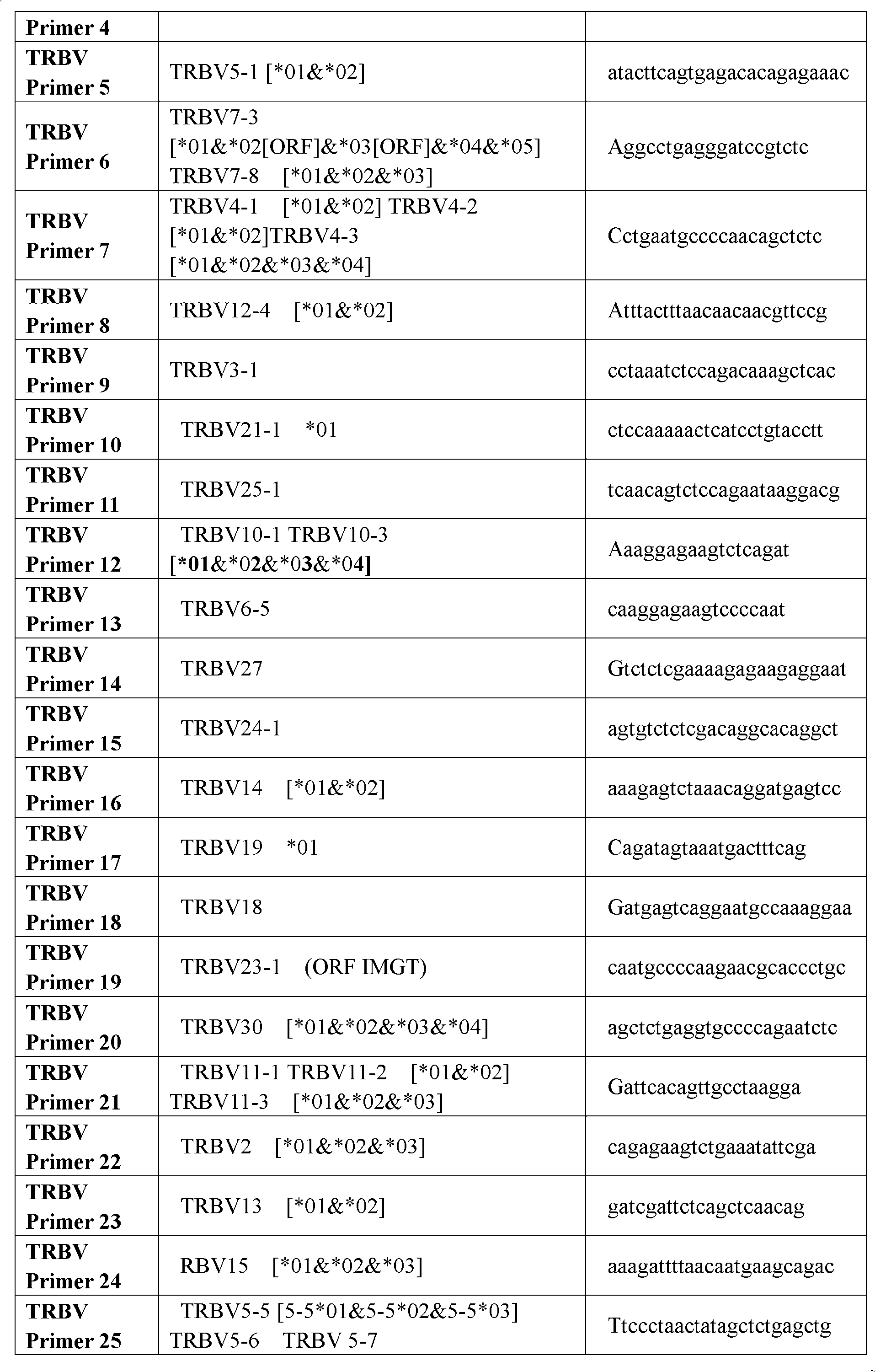
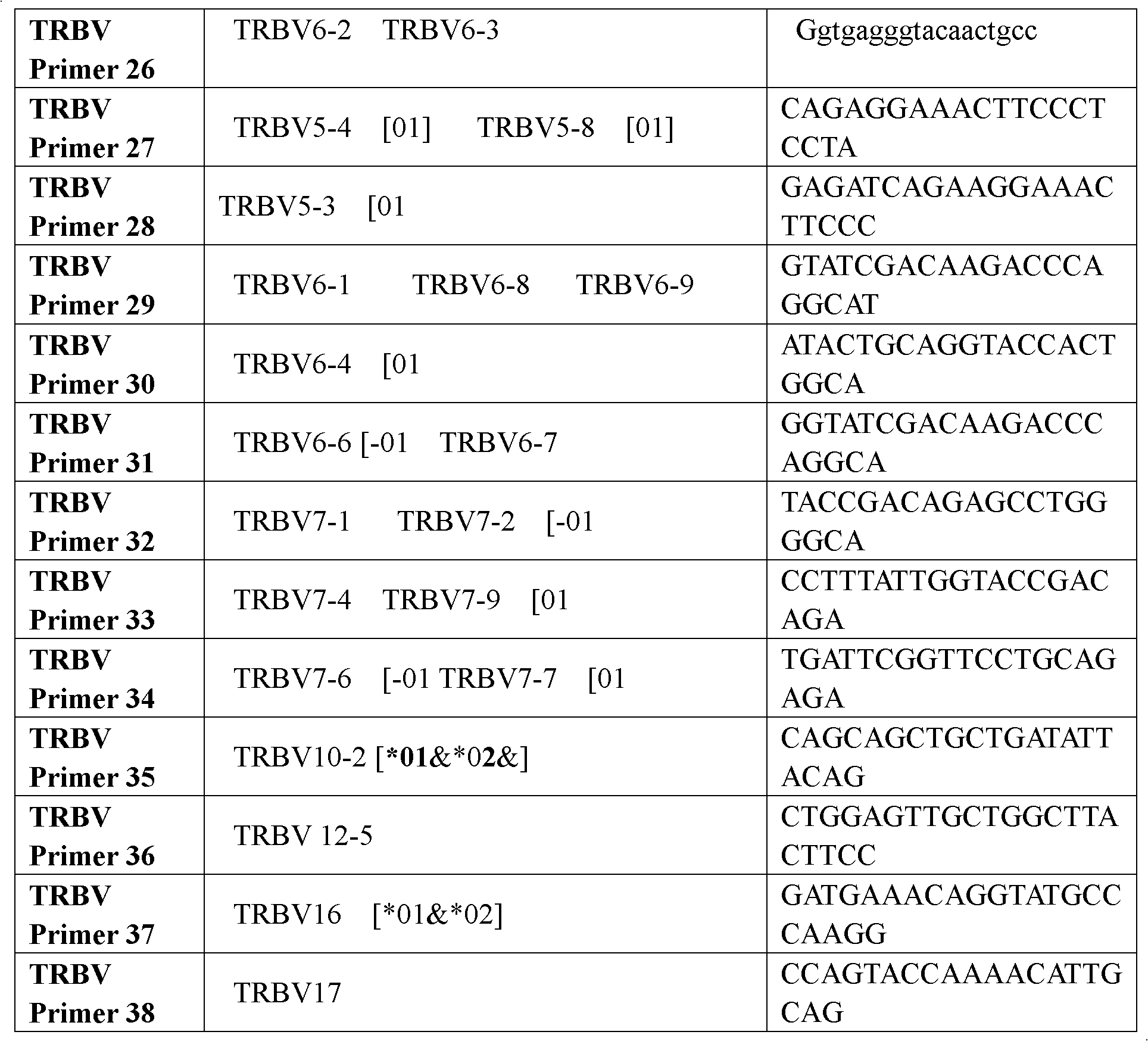
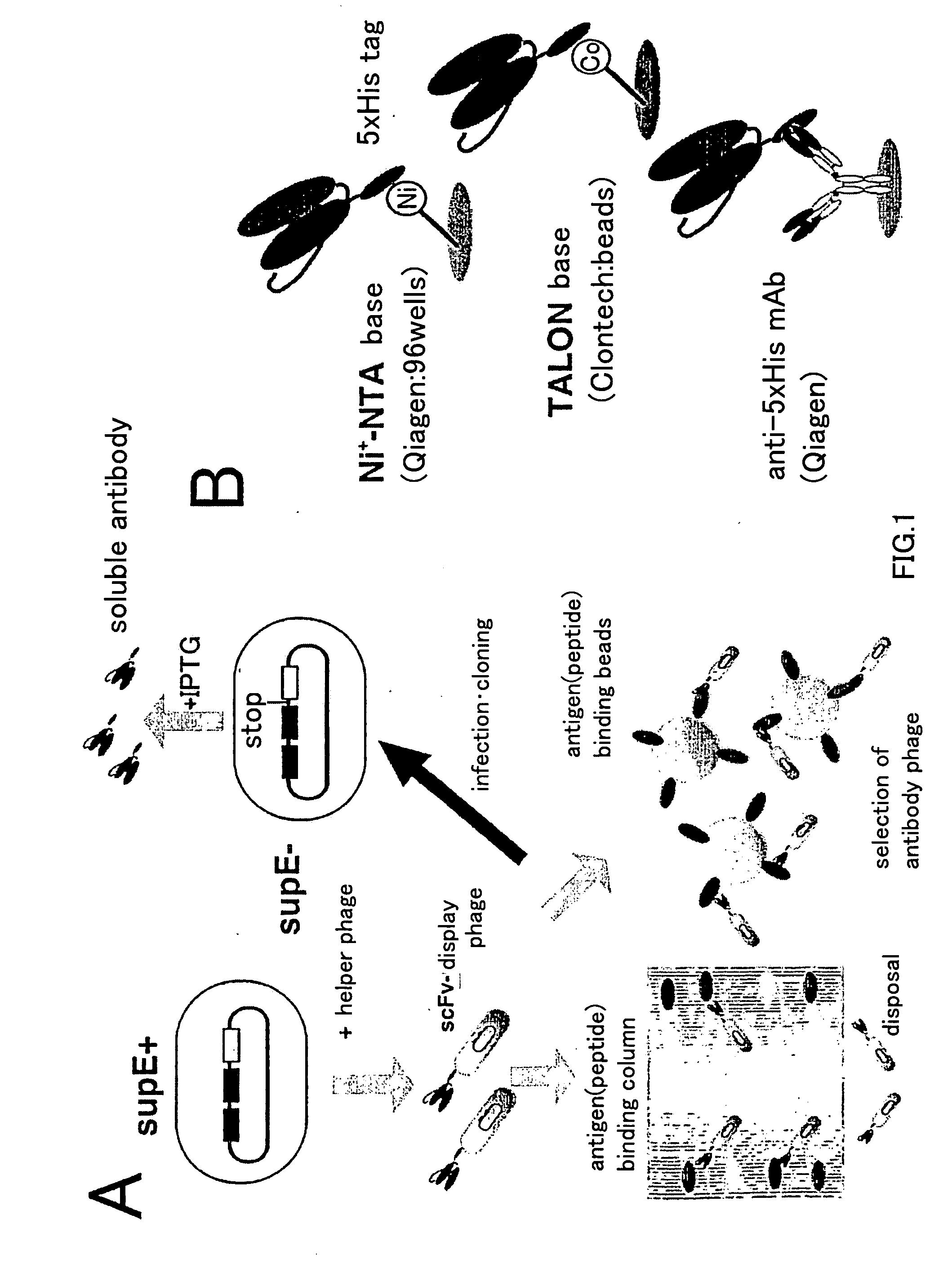
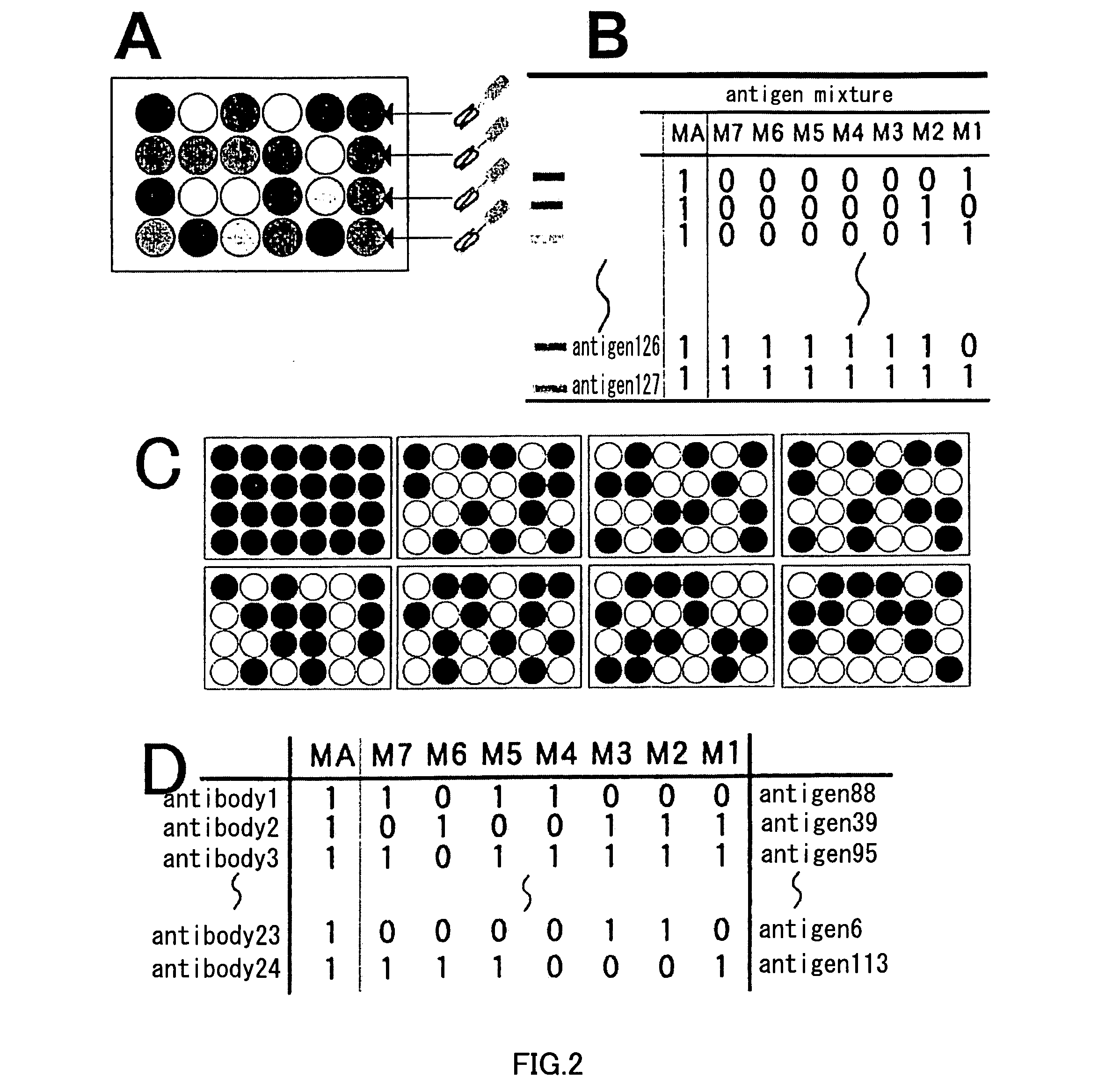
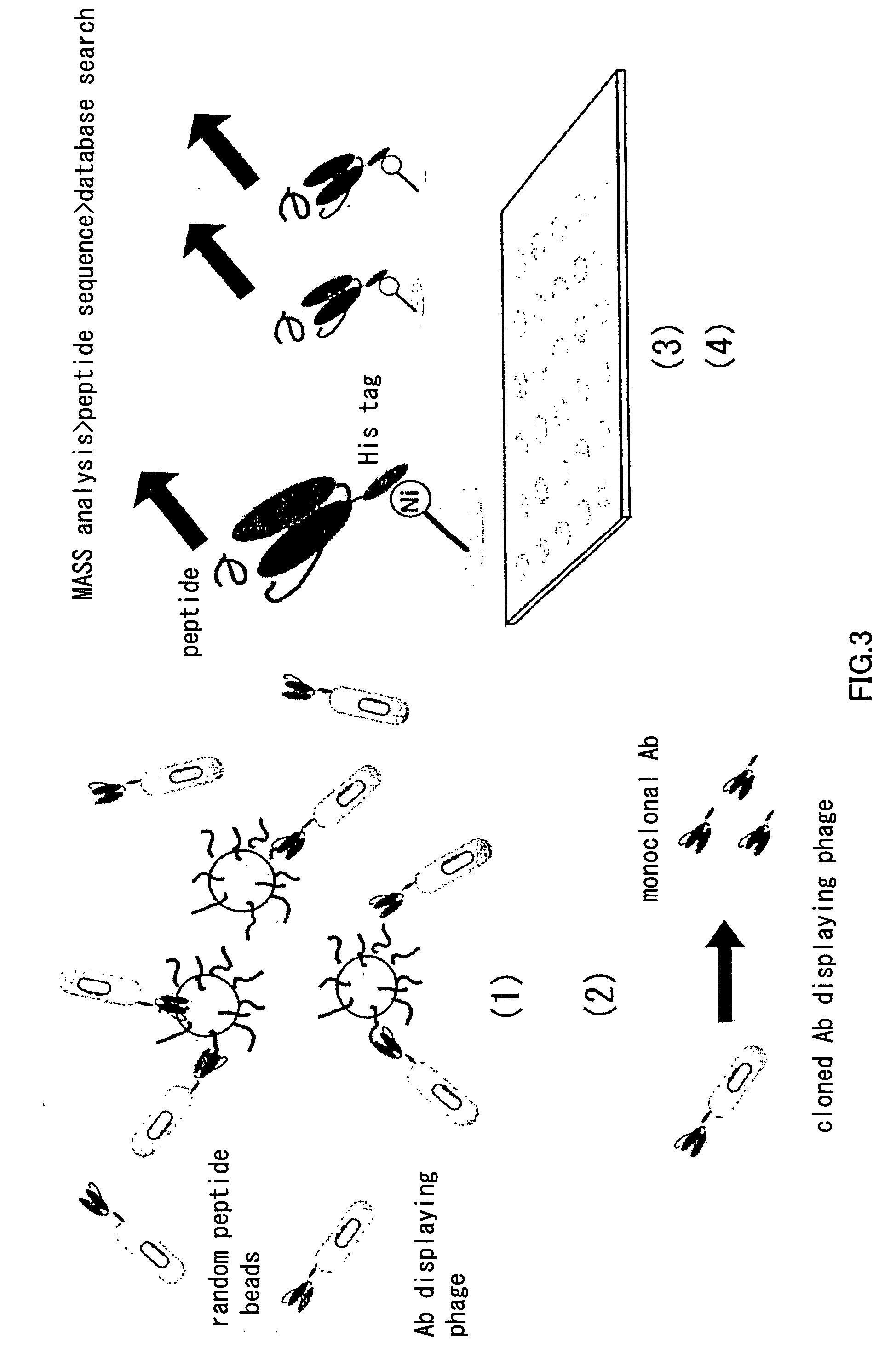
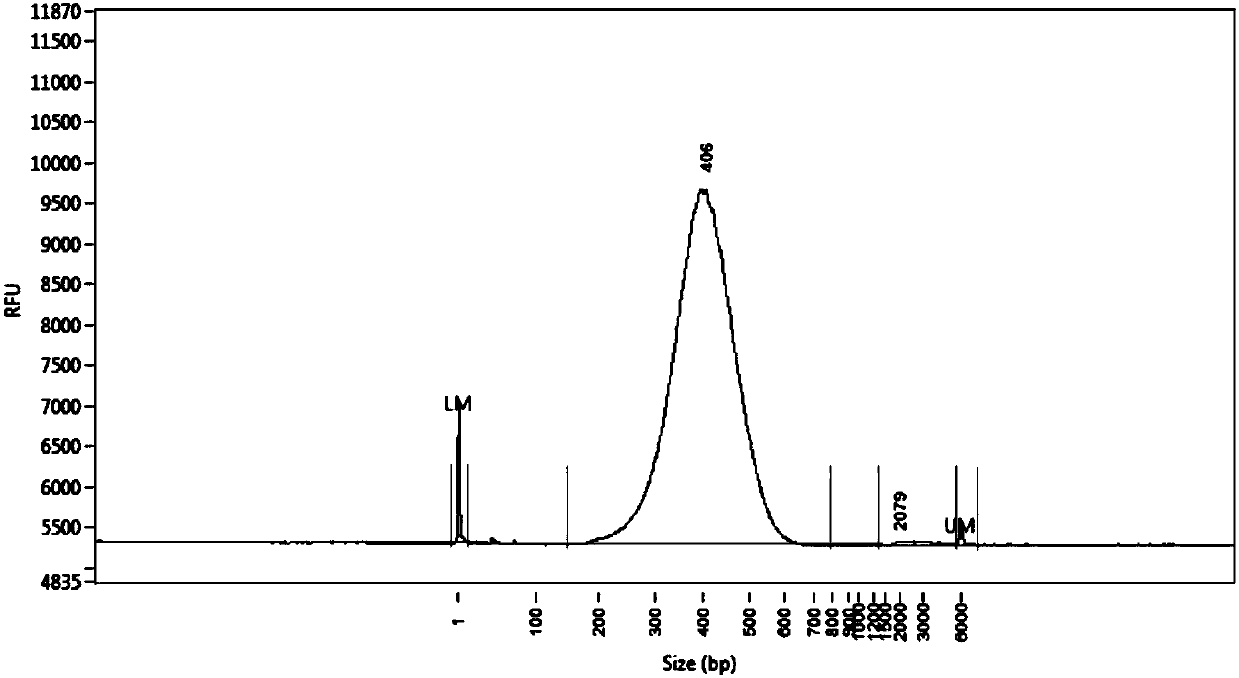
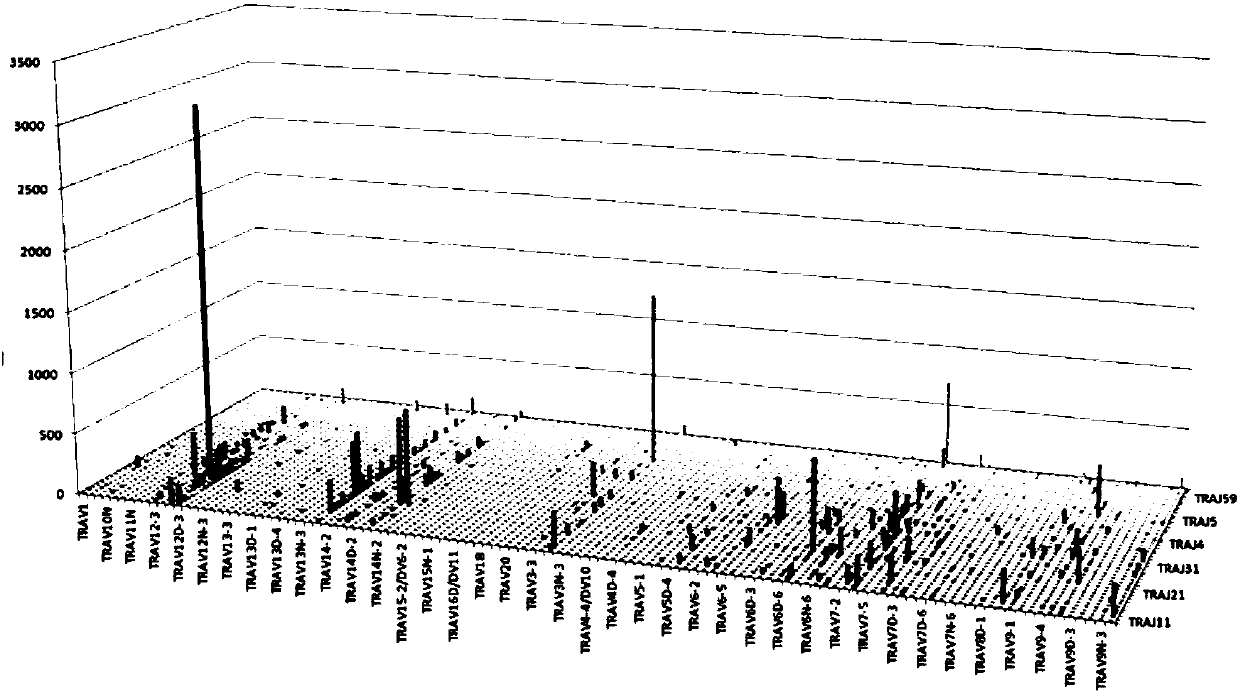
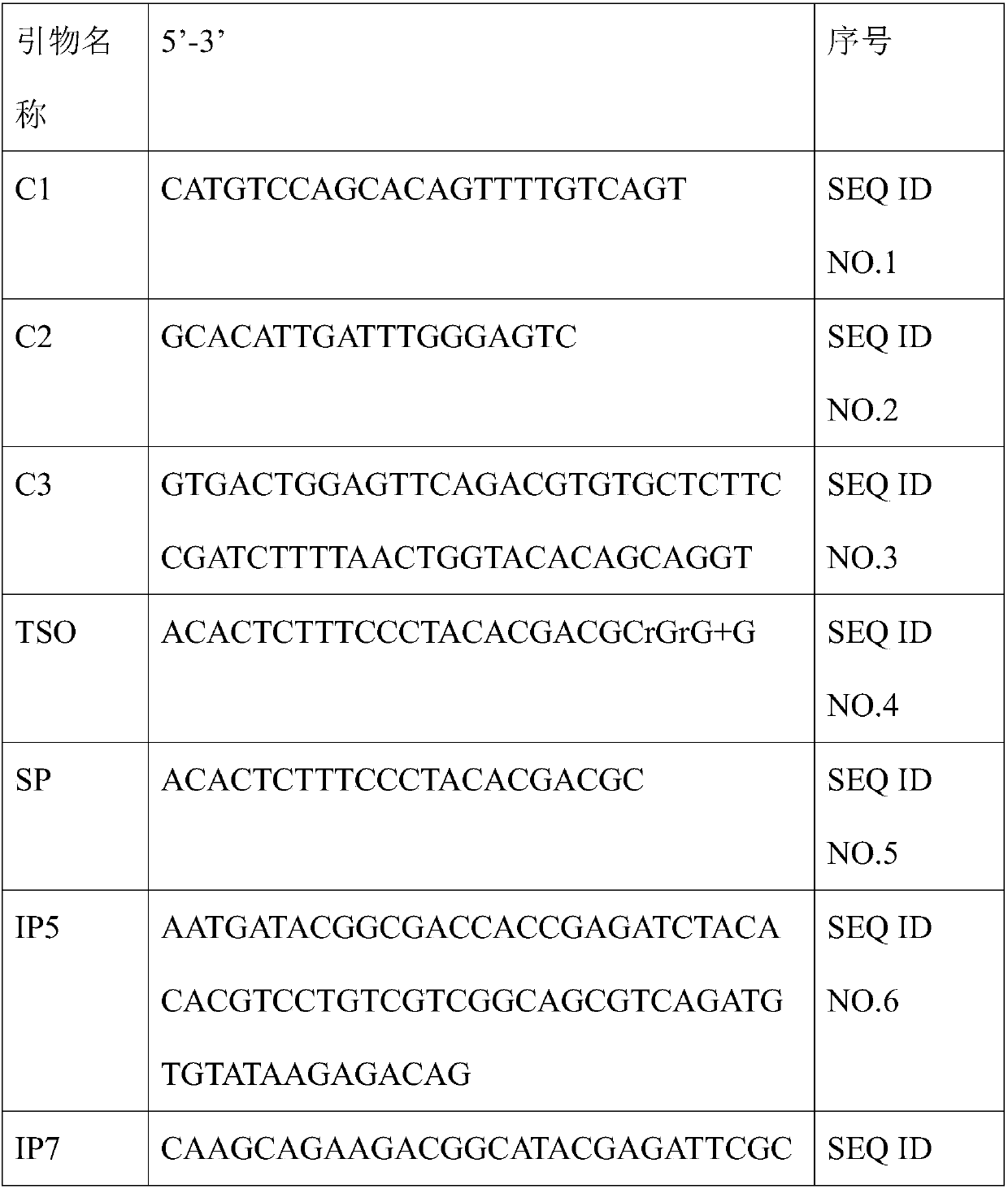
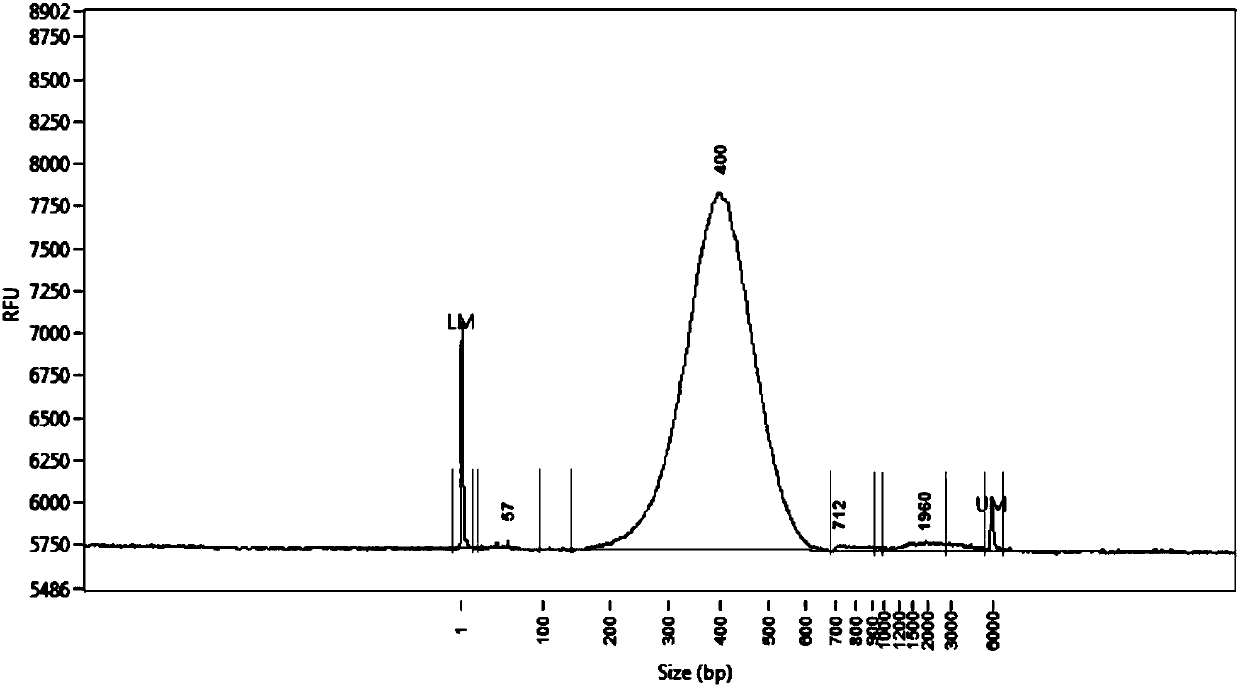
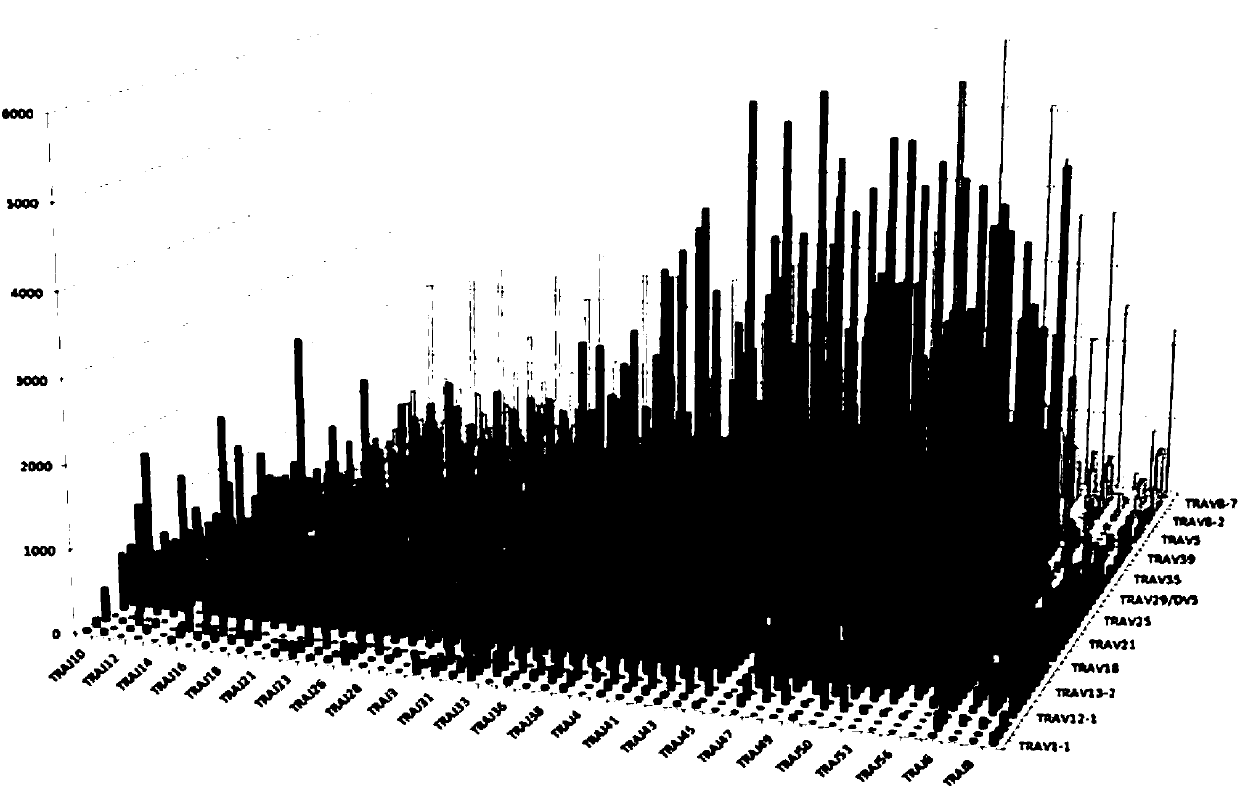
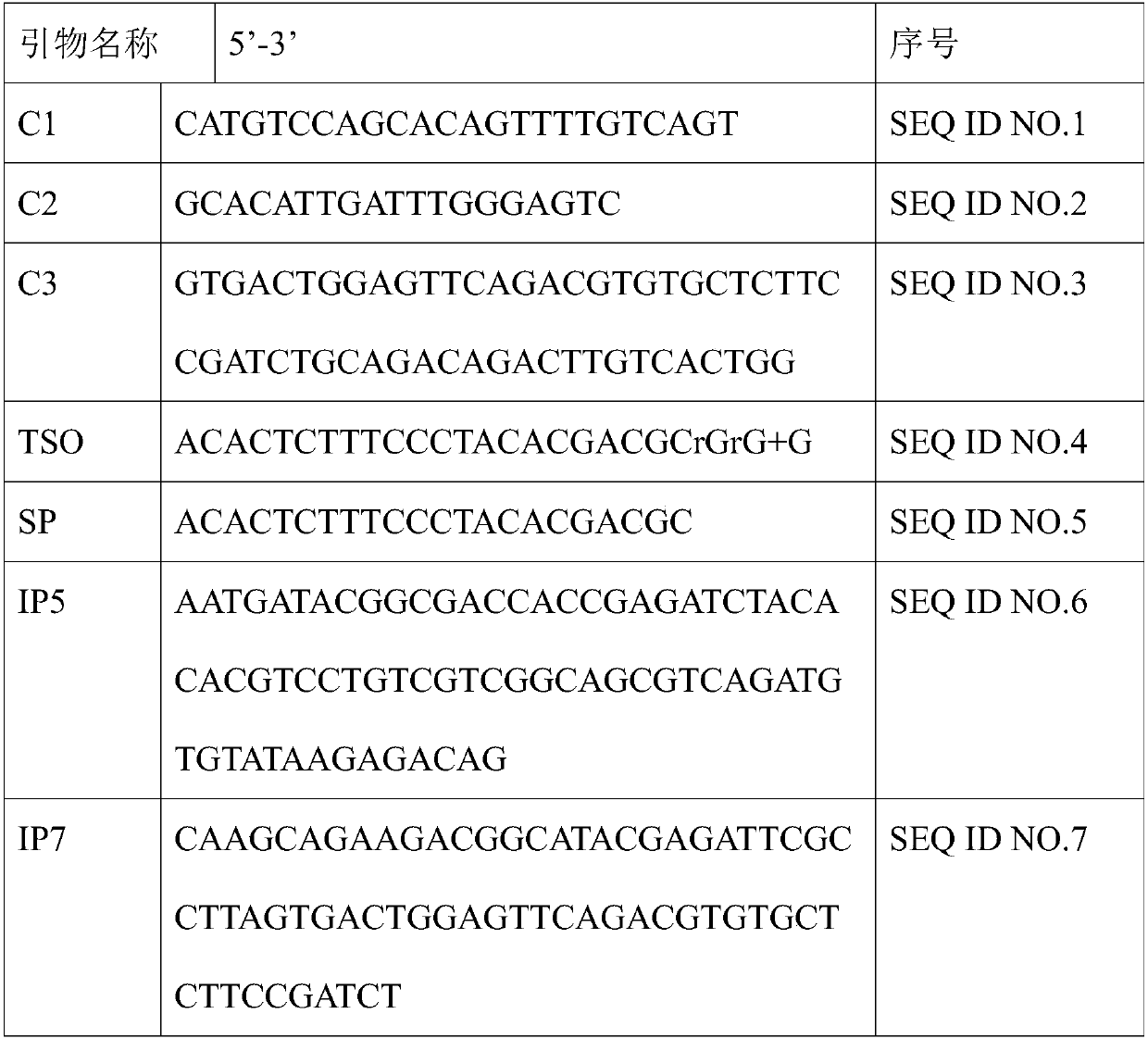
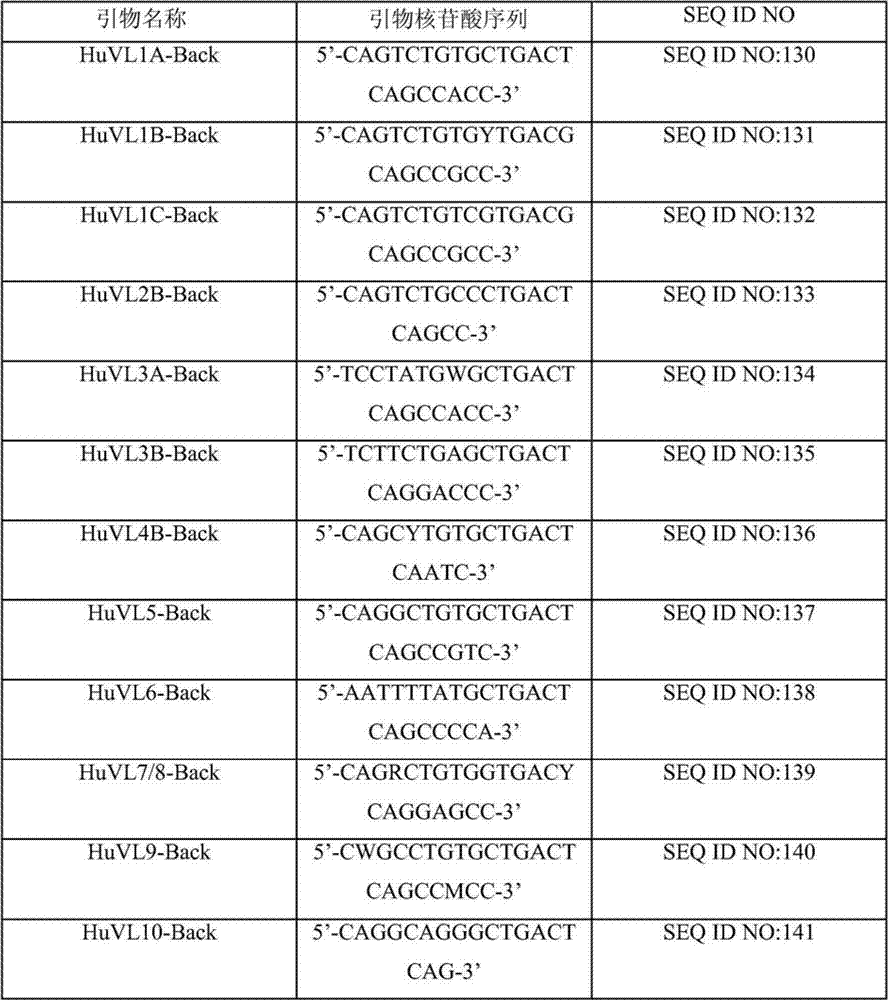
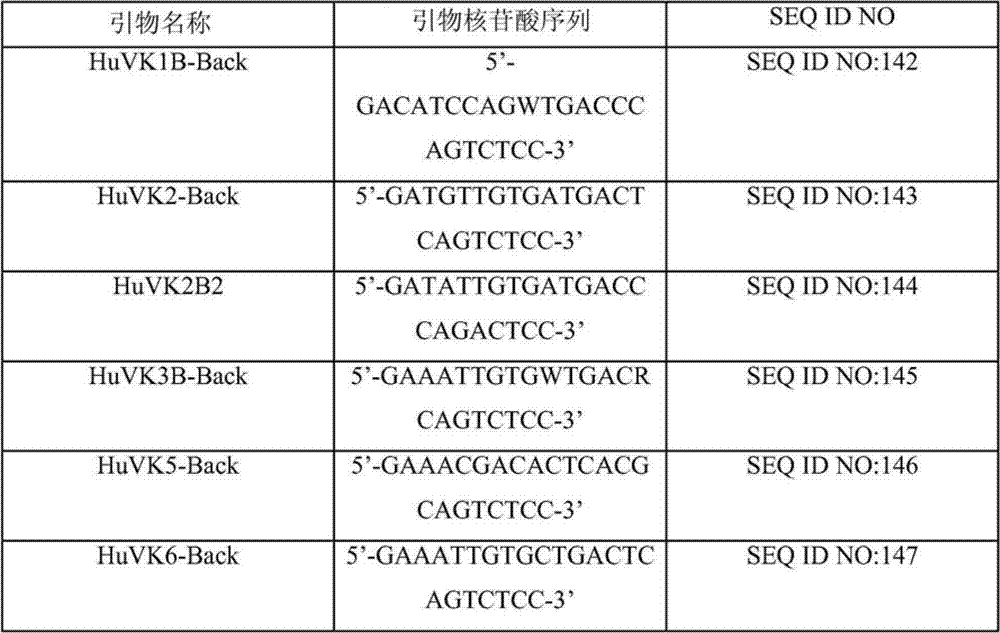
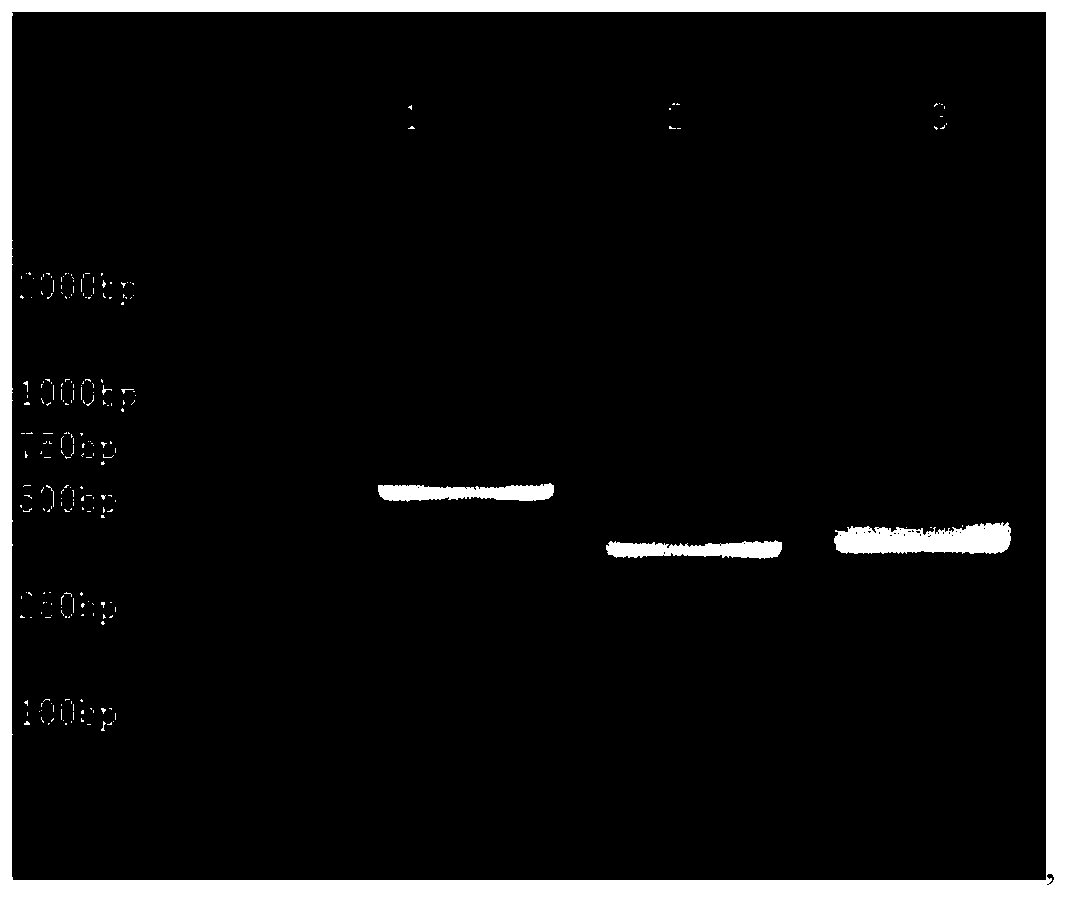
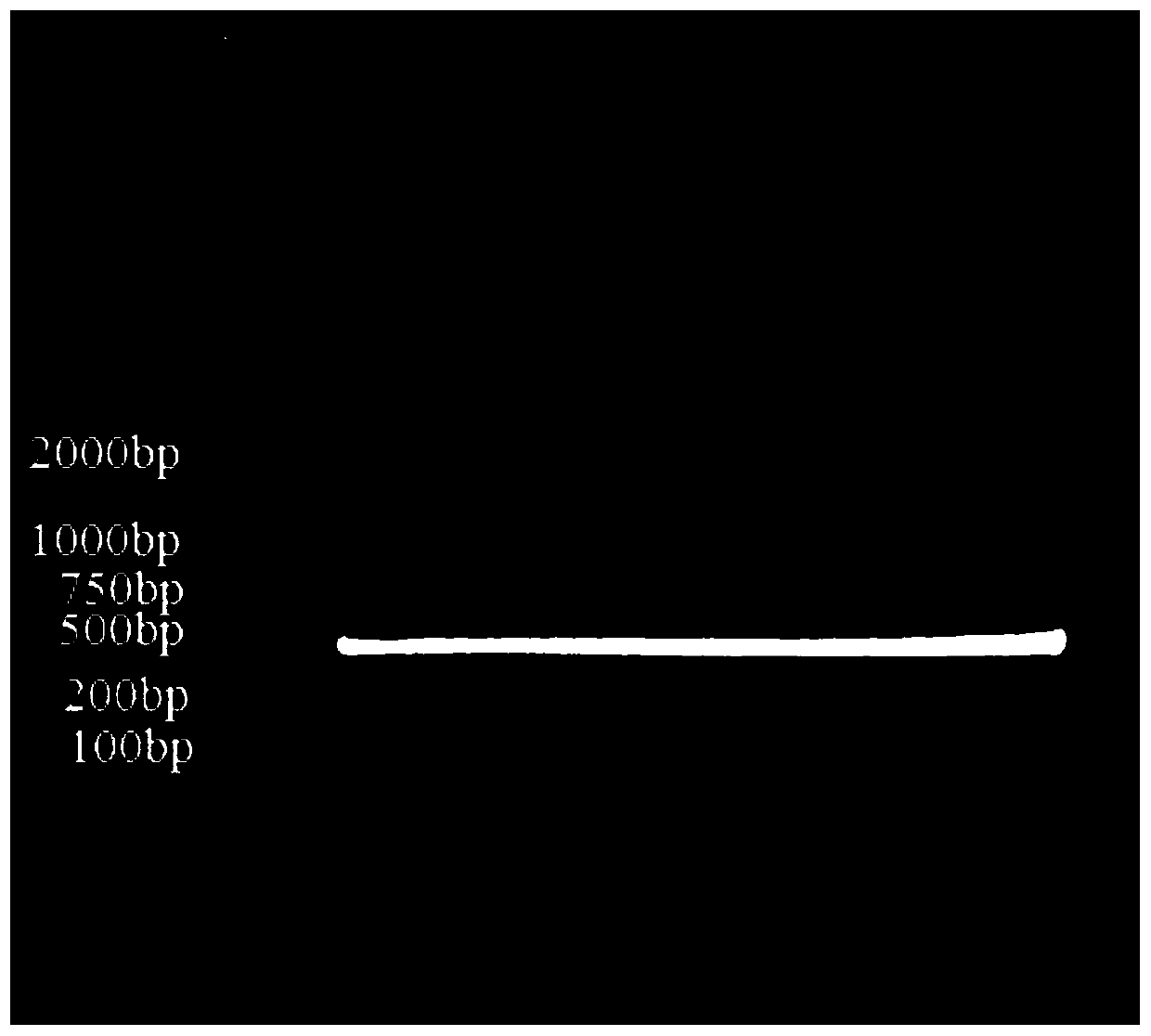
Primer group for constructing CDR3 region high-throughput sequencing library of human TCR[beta] and application of primer group
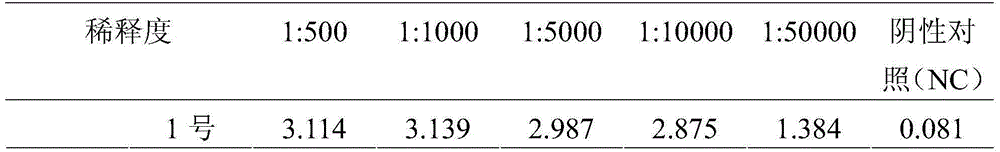
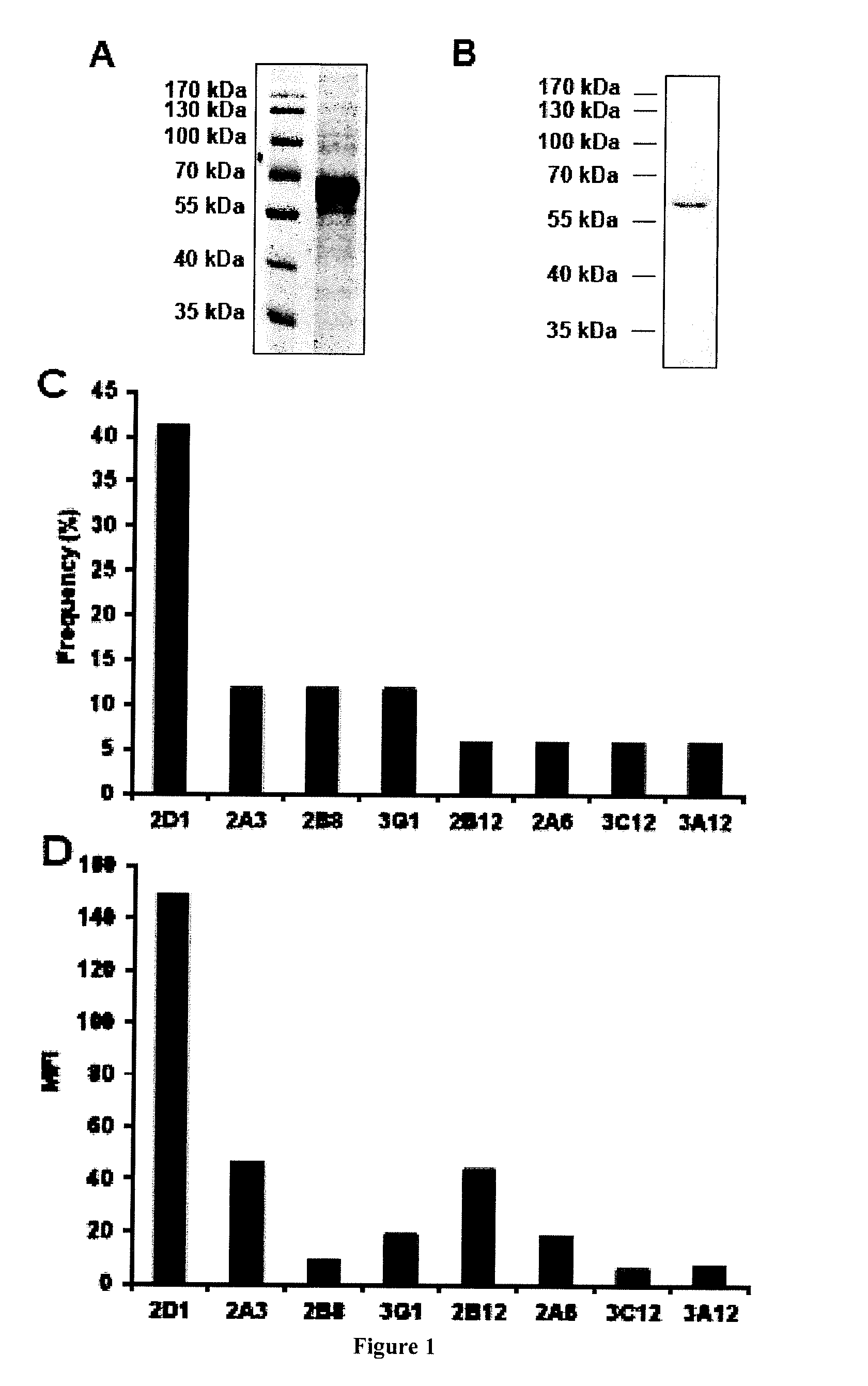
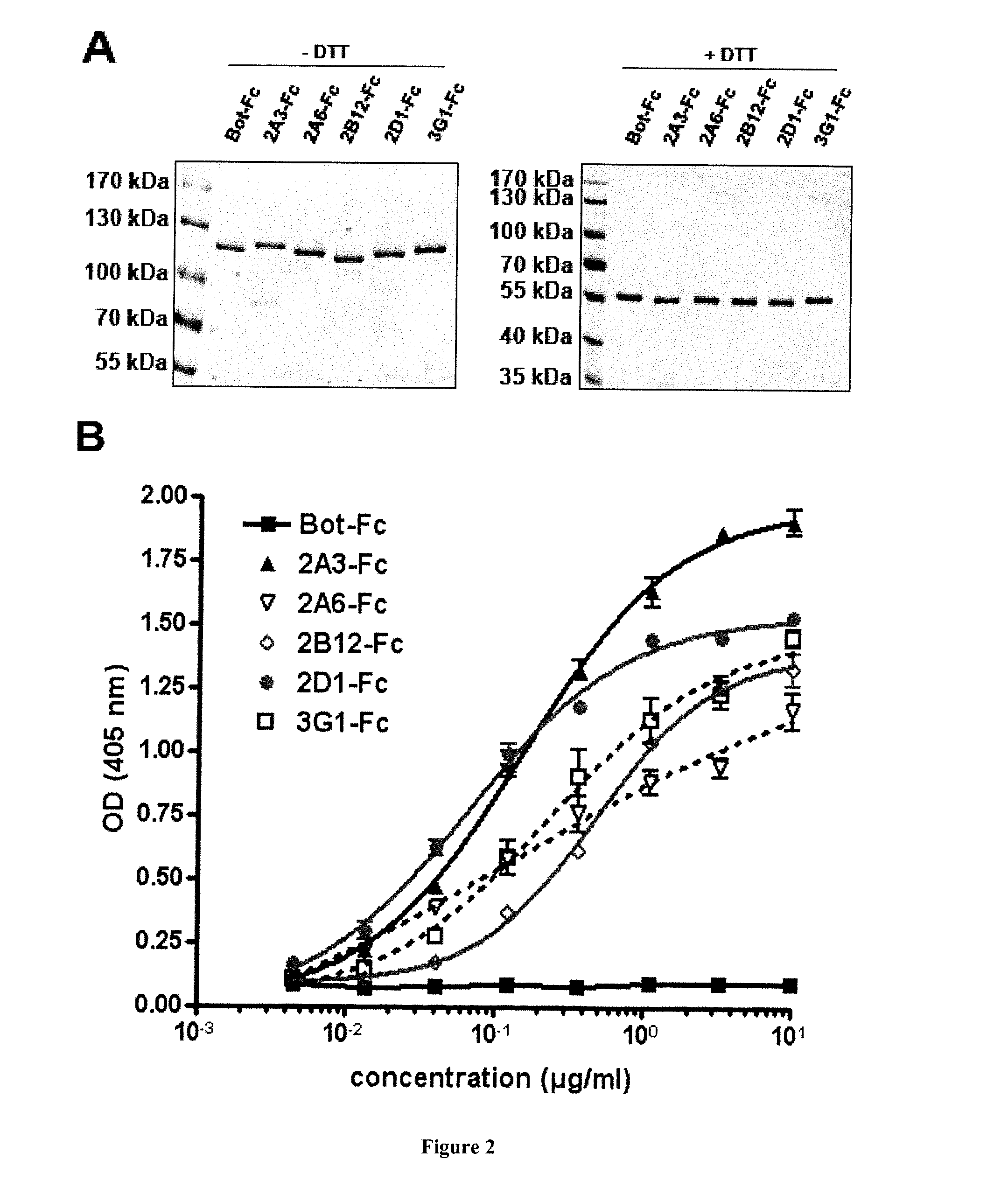
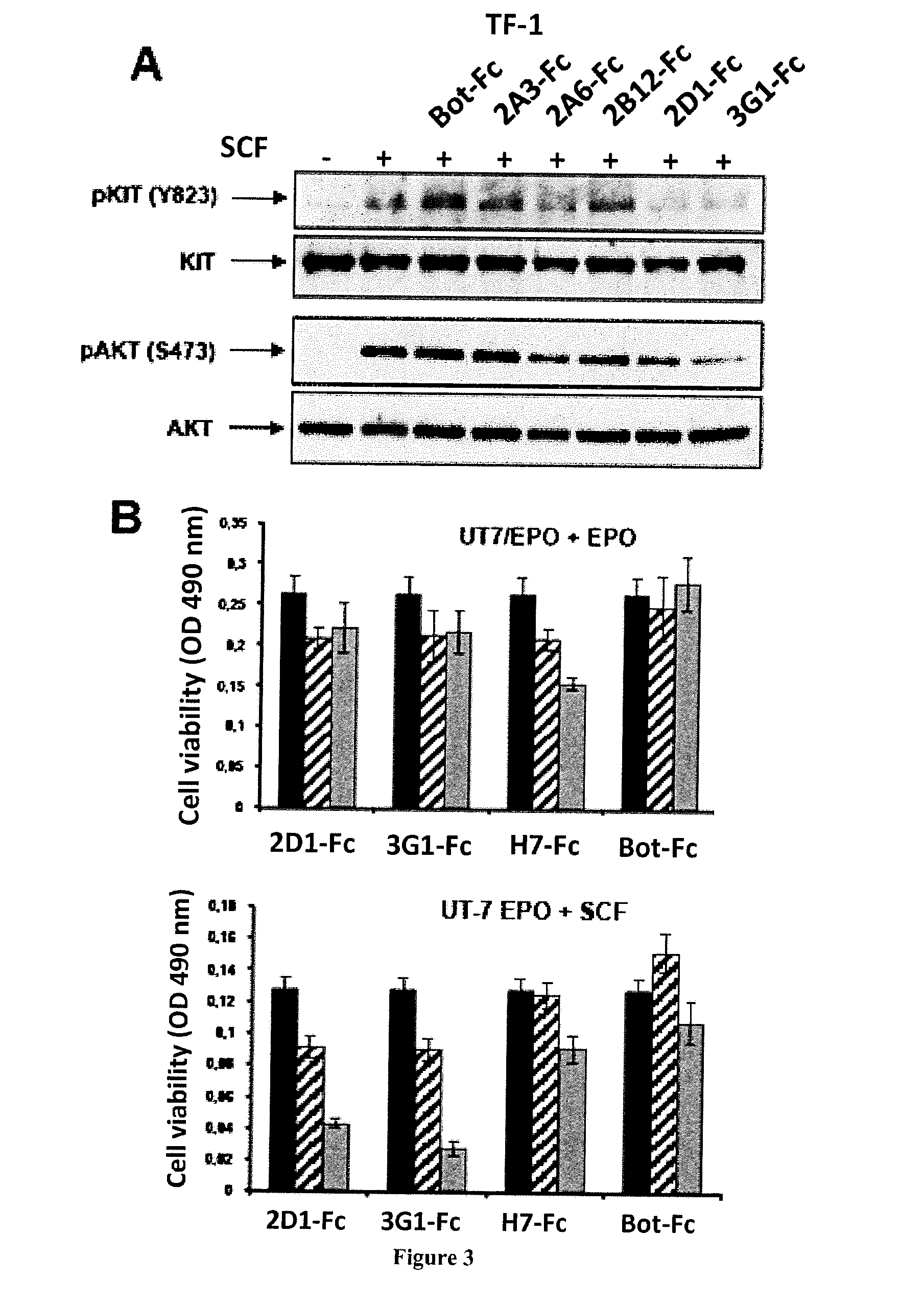
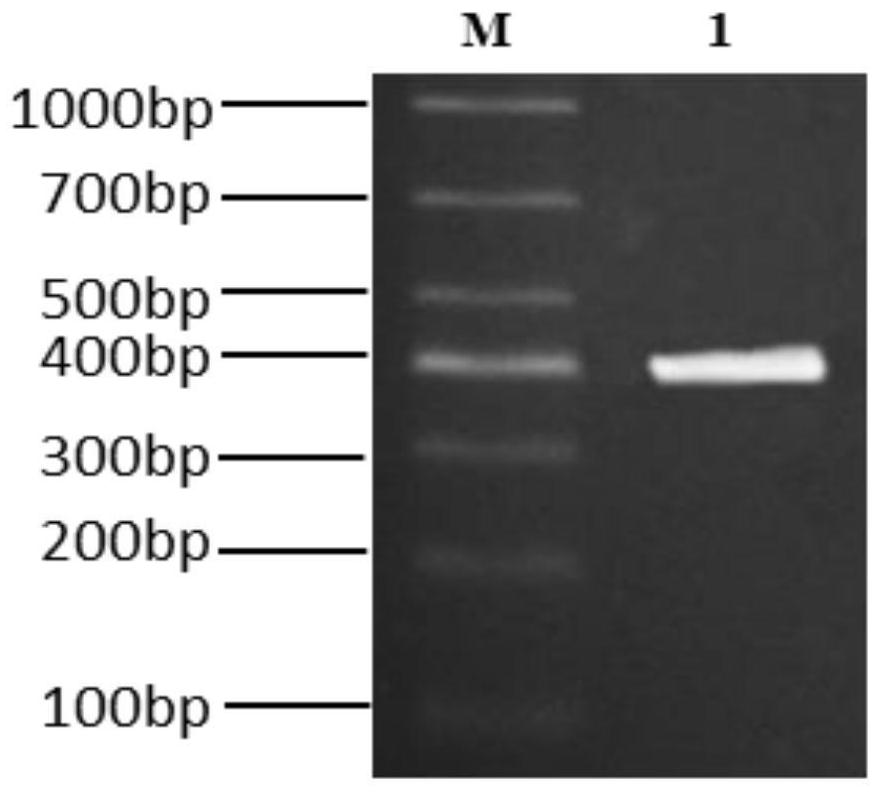
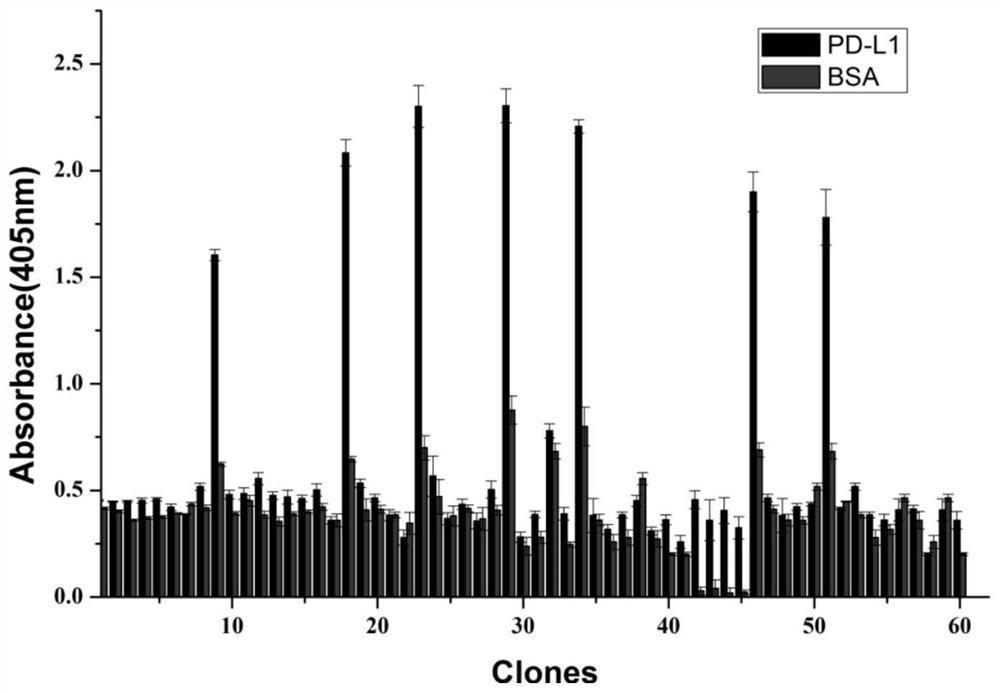
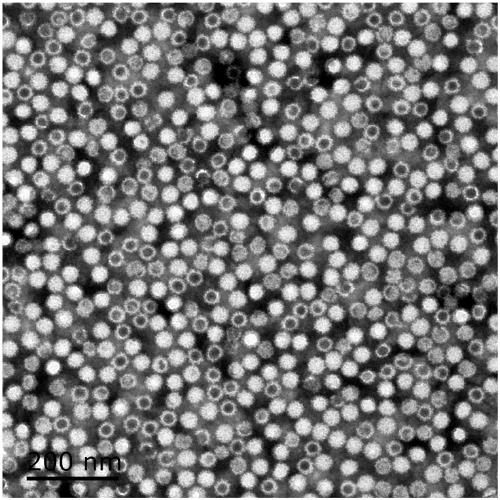
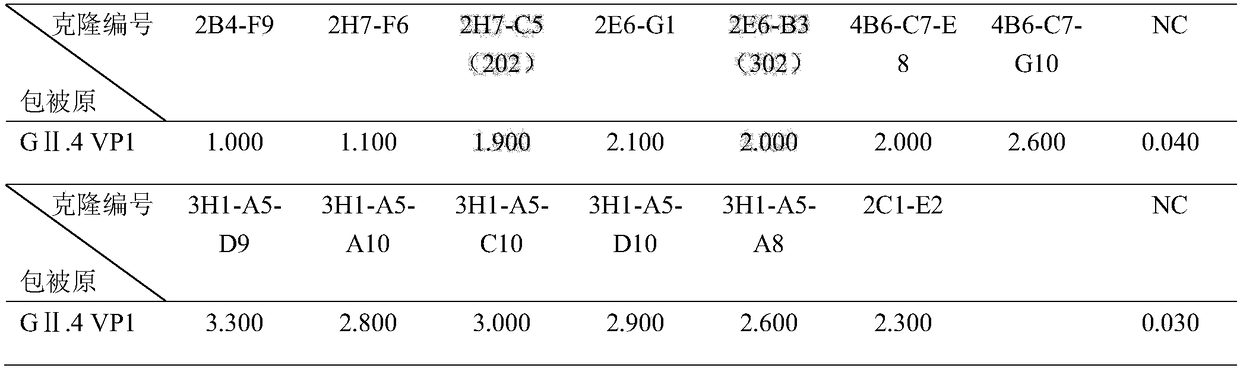
ActiveCN112143777AStable and accurate detectionThe experimental operation process is highly standardized and automatedMicrobiological testing/measurementLibrary creationLibrary preparationHigh throughput sequence
Owner:BEIJING IMMUPEUTICS MEDICINE TECH LTD
Humanized antibody or fragment thereof specific for cd3
ActiveCN107889492AImmunoglobulins against cell receptors/antigens/surface-determinantsMammal material medical ingredientsAntiendomysial antibodiesHeavy chain
The present invention provides a humanized antibody or fragment thereof specific for the antigen CD3, wherein said antibody or fragment thereof comprises a heavy chain variable domain comprising a CDR1 region of SEQ ID NO: 1, a CDR2 region of SEQ ID NO:2, and a CDR3 region of SEQ ID NO:3, and a light chain variable domain comprising a CDR1 region of SEQ ID NO:4, a CDR2 region of SEQ ID NO:5, and aCDR3 region of SEQ ID NO:6. In addition, the present invention provides a method for polyclonal stimulation of T cells comprising contacting a population of T cells with said anti-CD3 antibody, optionally with an anti-CD28 antibody, wherein said anti-CD3 antibody and optionally said anti-CD28 antibody are linked to particles. Further said anti-CD3 antibody can be used for reversible tagging of cells.
Owner:ミルテニイビオテックベーファーウントコーカーゲー
Agonistic 4-1bb monoclonal antibody
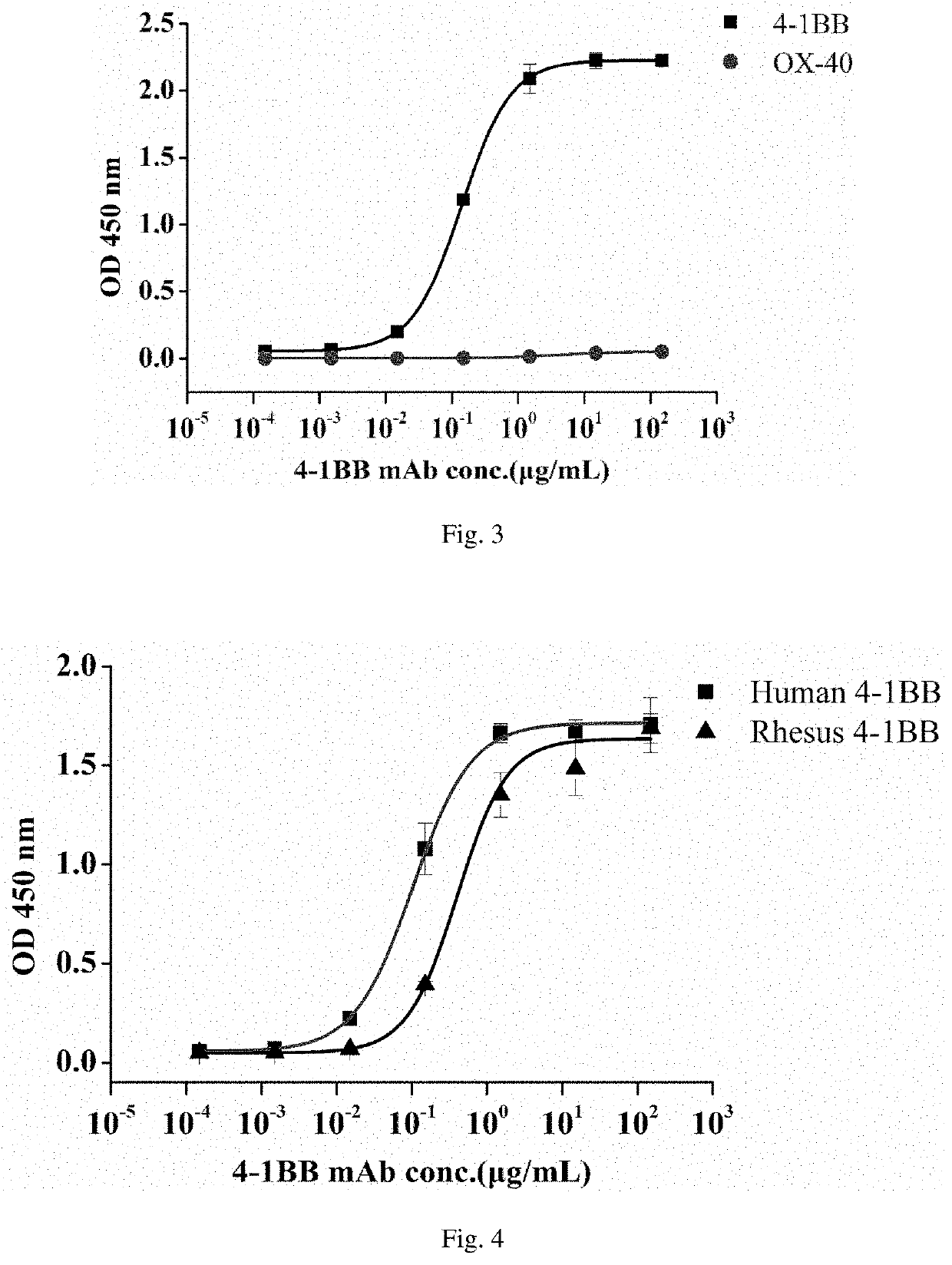
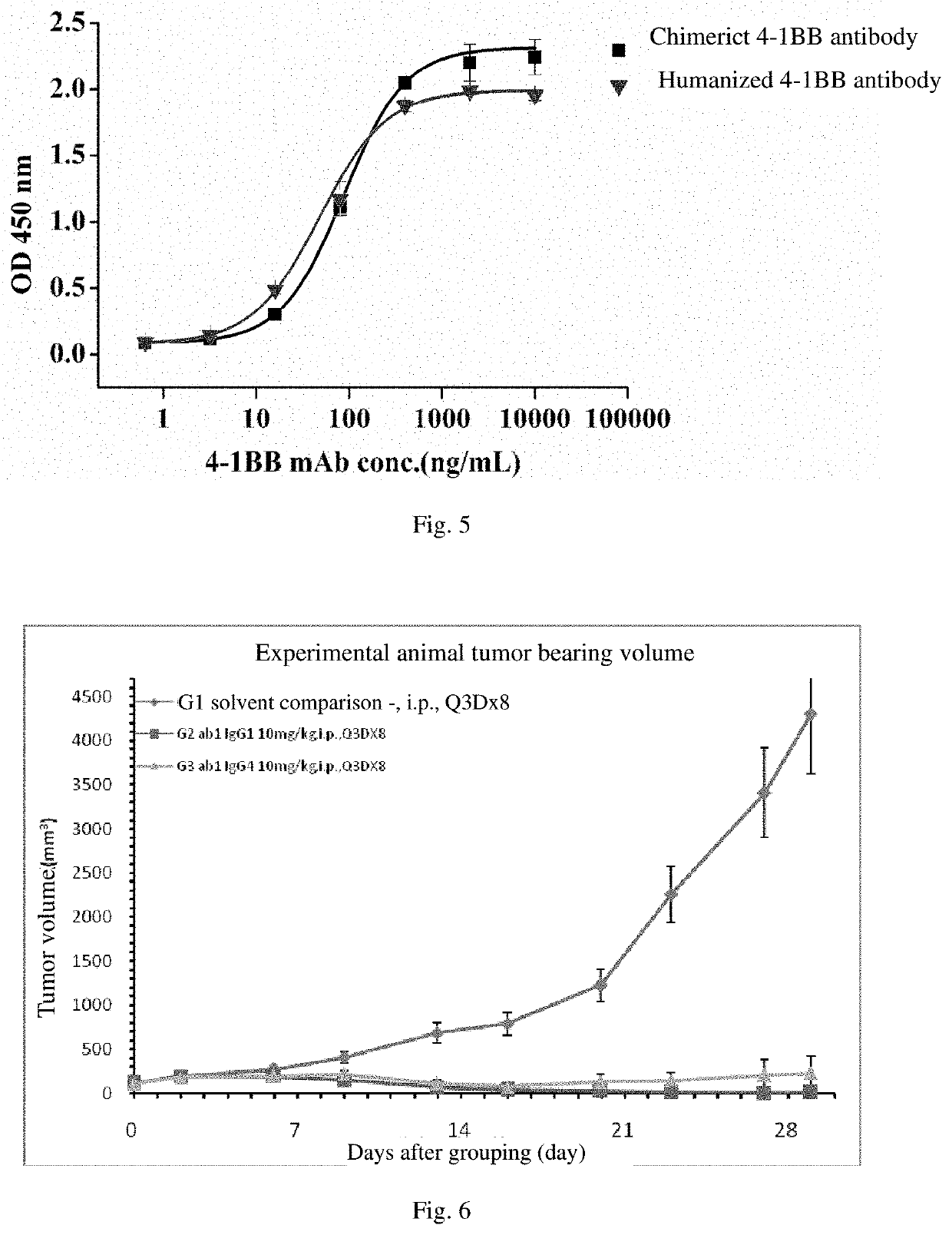
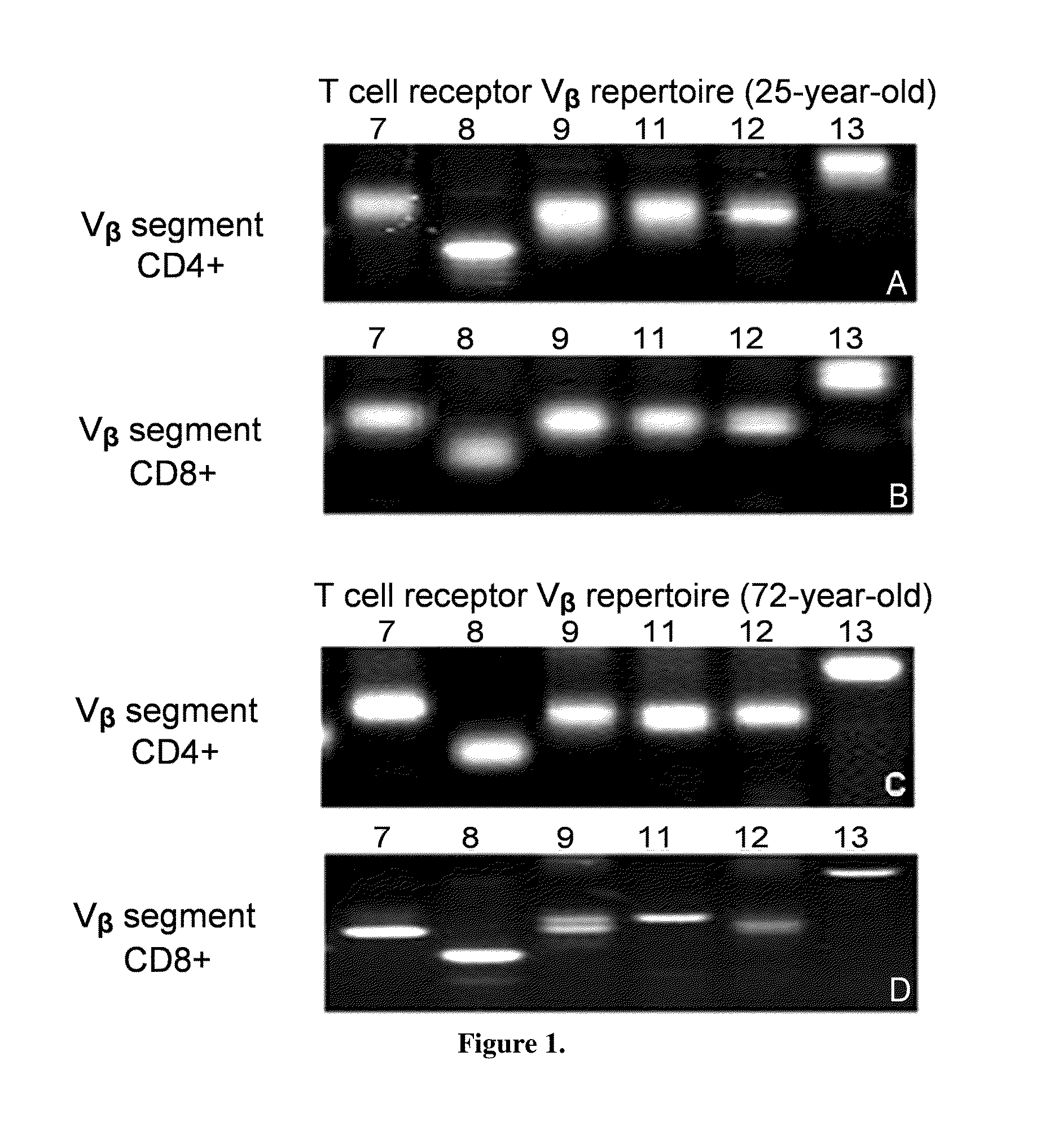
An agonistic 4-1BB monoclonal antibody or an antigen binding fragment thereof, comprising a heavy chain variable region and a light chain variable region, the heavy chain variable region comprising: a CDR1 region having an amino acid sequence as shown in SEQ ID NO: 1, a CDR2 region having an amino acid sequence as shown in SEQ ID NO: 2, and a CDR3 region having an amino acid sequence as shown in SEQ ID NO: 3, the light chain variable region comprising: a CDR1 region having an amino acid sequence as shown in SEQ ID NO: 4, a CDR2 region having an amino acid sequence as shown in SEQ ID NO: 5, and a CDR3 region having an amino acid sequence as shown in SEQ ID NO: 6. The monoclonal antibody is targeted towards h4-1BB, and specifically binds to h4-1BB so as to activate T cells. The monoclonal antibody has application potential for treating a variety of cancers by means of an immunomodulatory effect.
Owner:REYOUNG SUZHOU BIOLOGY SCI & TECH CO LTD
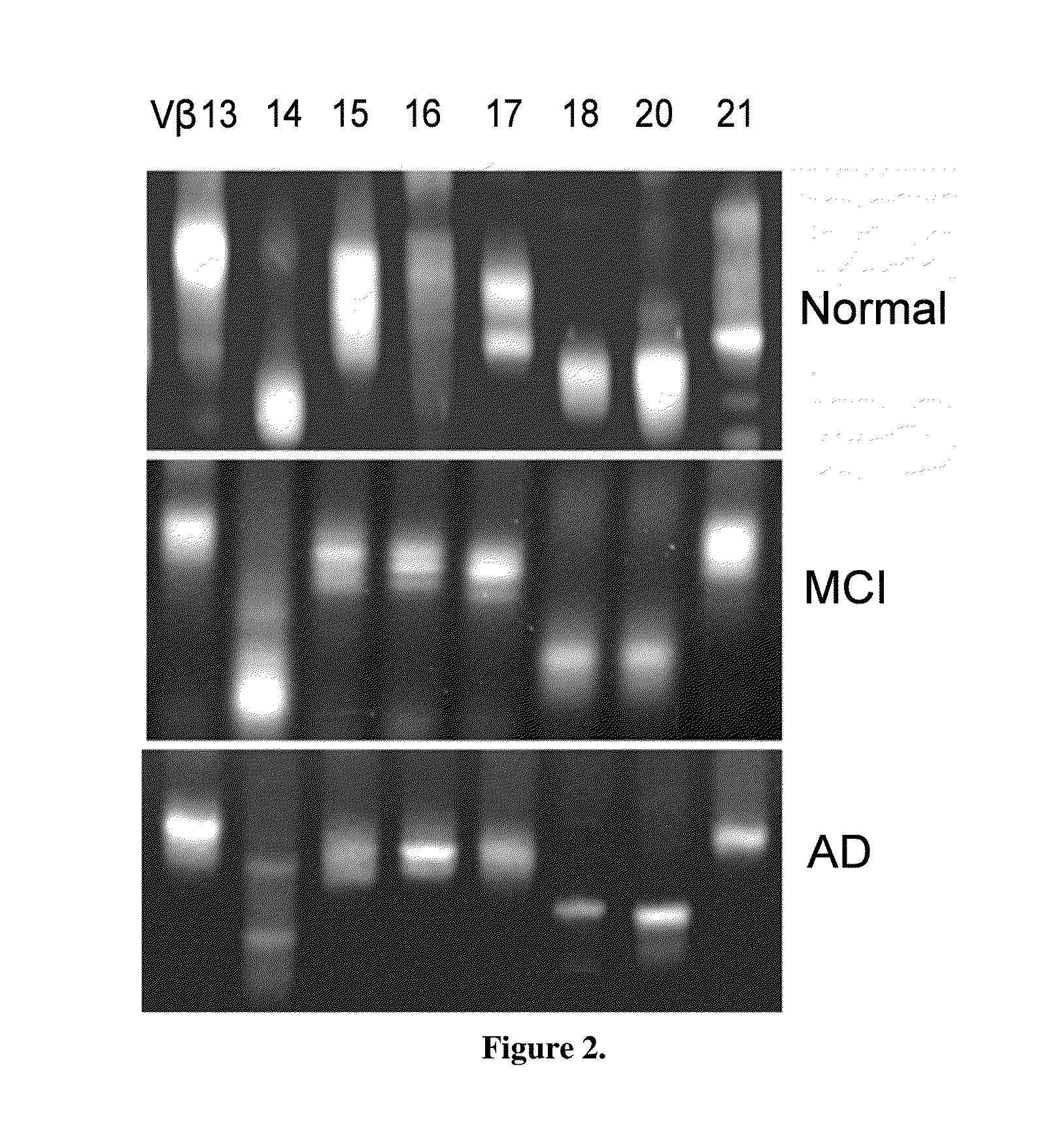
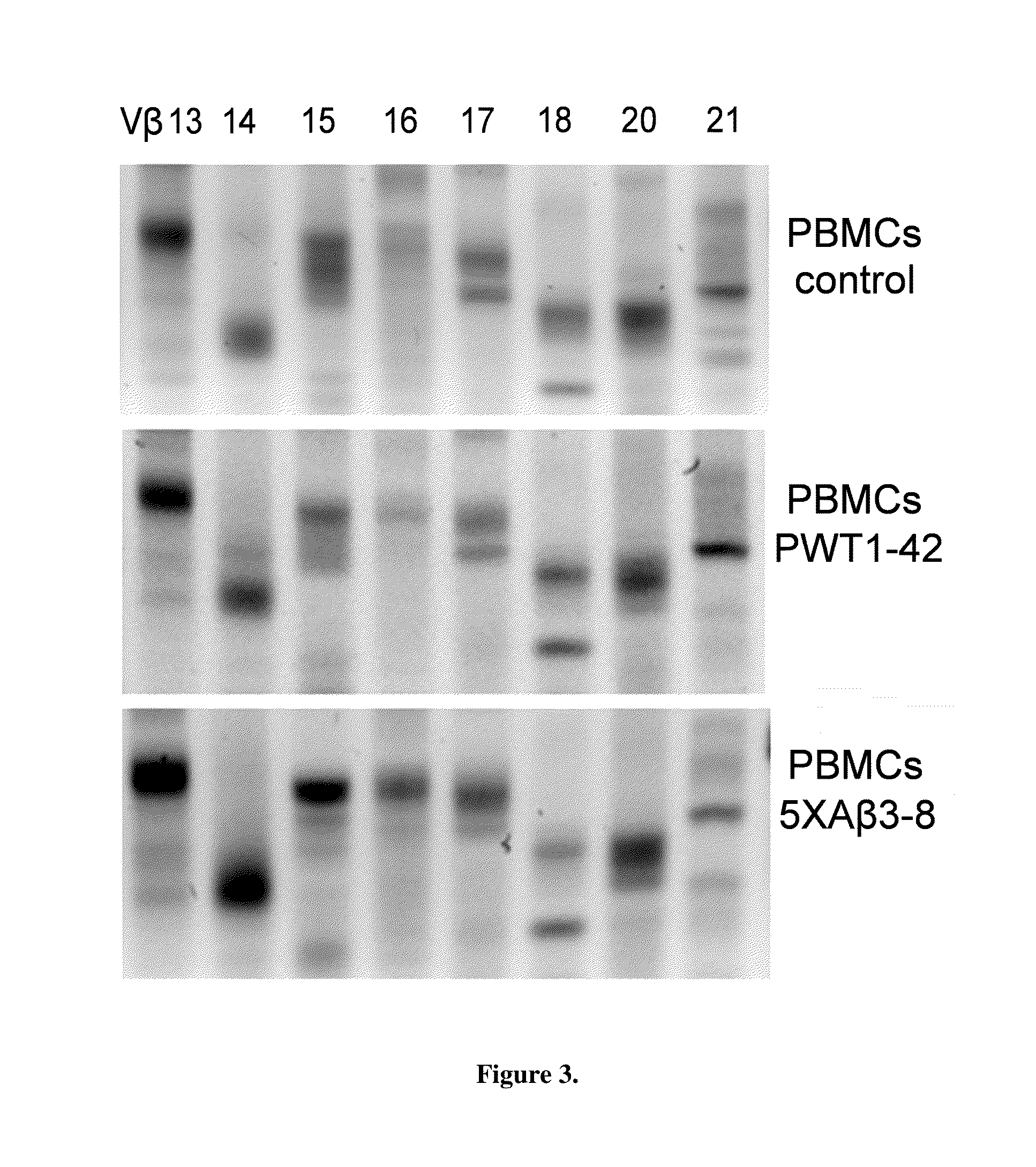
Method of diagnosing or assessing risk for Parkinson's disease or Alzheimer's disease using TCR clonality
ActiveUS8383347B1Amplifying inflammatory reactionExcessive activationMicrobiological testing/measurementFermentationNeuro-degenerative diseaseT cell
Changes in adaptive immune system were identified and correlated as a predictor of Aβ40 and Aβ42 and AD progression. T-cell, B-cell, TCR and BCR profiles were used to correlate clinical progression of AD. The CDR3 region was spectratyped, showing the clonality of the CDR3 region. This intrafamily gene fragment length profile was compared to age-matched controls, thereby indicating the existence of a neurodegenerative disease. The novel method is useful in diagnosing neurodegenerative disease, like Parkinson's disease, HIV-associated Dementia, or Alzheimer's disease. Moreover, this permits the clinical identification of patients at a very early stage of AD and / or monitoring the potential benefits of disease modifying therapeutics.
Owner:UNIV OF SOUTH FLORIDA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


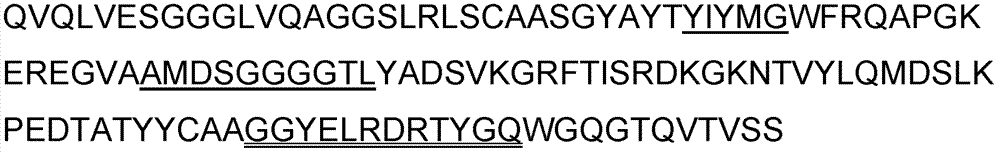







![Primer group for constructing CDR3 region high-throughput sequencing library of human TCR[beta] and application of primer group Primer group for constructing CDR3 region high-throughput sequencing library of human TCR[beta] and application of primer group](https://images-eureka.patsnap.com/patent_img/aa53ae03-e663-46aa-b806-2b038b5b3796/HDA0002637441920000011.png)
![Primer group for constructing CDR3 region high-throughput sequencing library of human TCR[beta] and application of primer group Primer group for constructing CDR3 region high-throughput sequencing library of human TCR[beta] and application of primer group](https://images-eureka.patsnap.com/patent_img/aa53ae03-e663-46aa-b806-2b038b5b3796/HDA0002637441920000012.png)
![Primer group for constructing CDR3 region high-throughput sequencing library of human TCR[beta] and application of primer group Primer group for constructing CDR3 region high-throughput sequencing library of human TCR[beta] and application of primer group](https://images-eureka.patsnap.com/patent_img/aa53ae03-e663-46aa-b806-2b038b5b3796/HDA0002637441920000021.png)