CD22 single domain antibody, nucleotide sequence, reagent kit, CAR-T virus vector and CAR-T cell
A nucleotide sequence, single-domain antibody technology, applied in nucleic acid vectors, DNA/RNA fragments, viruses/phages, etc., can solve the problems of difficult to achieve effective structure of CAR-T cells, low binding force, low affinity, etc. To achieve the effect of good killing of tumor cells, low production cost and high expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
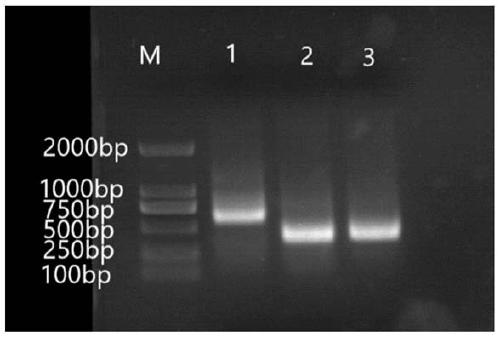
[0032] Example 1 Anti-CD22 Antigen Specific Antibody Library Construction
[0033] It should be noted that the CD22 antigen used in this example was purchased from Beijing Sino Biological, the article number is 11958-H08H1. The QIAGEN kit is the QIAamp RNA Blood Mini Kit (50) from Beijing Yaanda Biotechnology Co., Ltd., Cat. No. 52304. The cDNA synthesis kit is MiniBESTAgarose Gel DNA Extraction Kit Ver.4.0 from TAKARA Company.
[0034] 1. Immunization experiment.
[0035] 1) Select a healthy alpaca as the immune target;
[0036] 2) Using 2 mg CD22 antigen mixed with an equal volume of Freund's adjuvant to immunize a healthy alpaca, a total of 6 immunization experiments were carried out;
[0037] 3) After the 4th immunization experiment, the alpaca serum was sampled, and its antigen immune titer was determined, until the measured immune titer of the alpaca serum reached more than 10,000 times, 100ml of alpaca whole blood was taken, and isolate lymphocytes;
[0038] 4) lys...
Embodiment 2
[0067] The screening of embodiment 2 specific phage
[0068] 1) Preliminary preparation. Prepare 5*10 CD22-negative control cell lines and modified CD22-positive cell lines respectively 6 , use CPBSH or 2% BSA-PBS to incubate on a drum at room temperature for 2 hours, and centrifuge for later use.
[0069] 2) Screening. The phage (5*10 11 ~1*10 12 ) into negative cells, CPBS or 2% BSA-PBS to 0.5ml, incubate on a drum at room temperature for 2 hours; take supernatant, add to positive cells, CPBS or 2% BSA-PBS to 0.5ml , incubate on a drum at room temperature for 2 hours; so that the outer shell of the phage can specifically secrete CD22 antibody and bind to CD22 antigen; wash 5 times with PBST, wash 3 times with PBS, centrifuge, and discard the supernatant, so that no binding The phages were washed away.
[0070] 3) Titer determination. The above cell-bound phages were resuspended, and 2ml of TG1 in the logarithmic growth phase was taken, infected, and allowed to stand a...
Embodiment 3
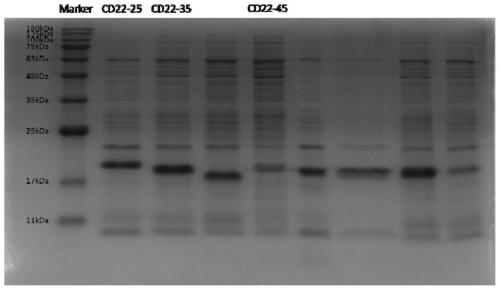
[0076] Example 3 Screening of specific positive monoclonal antibodies
[0077] 1) Screen the highly expressed recombinant plasmids.
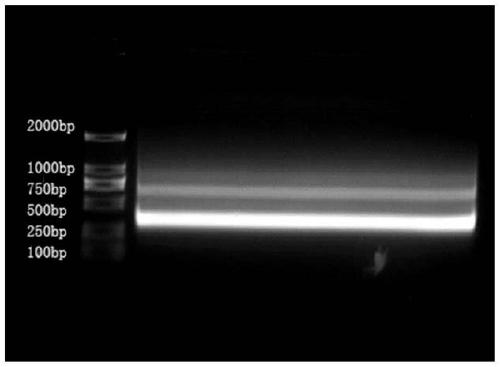
[0078] The specific CD22 antibody gene fragment obtained in Example 2 was amplified by PCR to obtain PCR products with restriction endonucleases BbsI and BamHI sites; the above PCR products and pSJF2 were treated with restriction endonucleases BbsI and BamHI respectively Vector; T4 ligase is used to connect the above-mentioned digested gene fragments and recombine them to obtain a recombinant plasmid sdAb-pSJF2 capable of high-efficiency expression in Escherichia coli.
[0079] 2) Screen CD22 positive cloned antibodies.
[0080] Pick a single colony from the agar plate where the colony grows, inoculate it in a 96-well deep-well culture plate containing Amp-containing 2YT liquid medium, and cultivate it for 4 hours; inoculate the single clones one by one in the numbered cells separated by small grids On the LB solid plate containing Amp; add IP...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com