Iodine resistance screening kit for papillary thyroid carcinoma
A thyroid cancer and kit technology, applied in the field of cancer diagnosis, can solve the problems of tissue and organ metastasis, ineffective radioactive iodine 131 treatment, affecting the quality of life and survival rate of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: kit of the present invention
[0025] This embodiment takes the PCR screening kit as an example for introduction.
[0026] 1. Composition of the kit
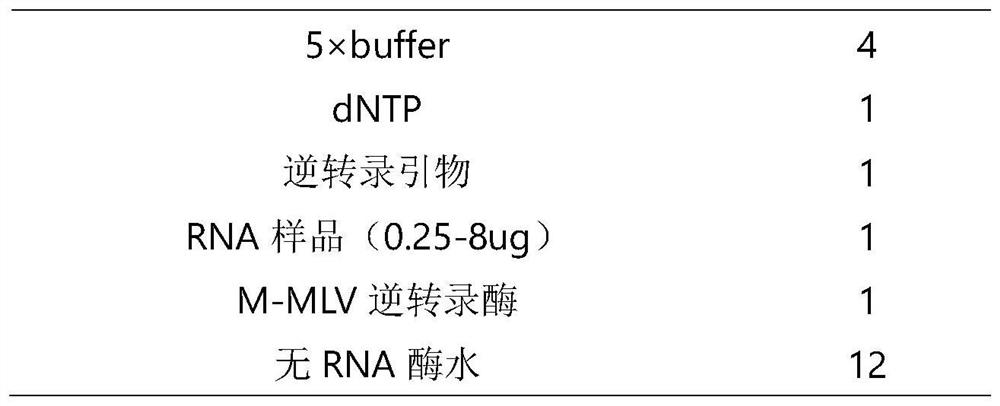
[0027] 1.1 Reverse transcription reagents
[0028] (1) MicroRNA Stem-loop reverse transcription primer
[0029] The sequence (SEQ ID NO.1) is:
[0030] 5'-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCACAAA-3' (SEQ ID NO. 1).
[0031] (2) Other conventional reagents required for reverse transcription
[0032] Including dNTP, 5×buffer, M-MLV reverse transcriptase; it can be a conventional reverse transcription reagent in the field.
[0033] 1.2 Real-time fluorescent quantitative PCR reagents
[0034] (1) Quantitative PCR primers
[0035] The sequences are:
[0036] Forward primer (SEQ ID NO.2): 5'-GGGTAGCAGCACATAATGG-3'(SEQ ID NO.2);
[0037] Reverse primer (SEQ ID NO. 3): 5'-CTCAACTGGTGTCGTGGA-3' (SEQ ID NO. 3).
[0038] (2) Real-time fluorescent quantitative PCR master mix
[0039] TB Green Premix...
experiment example 1
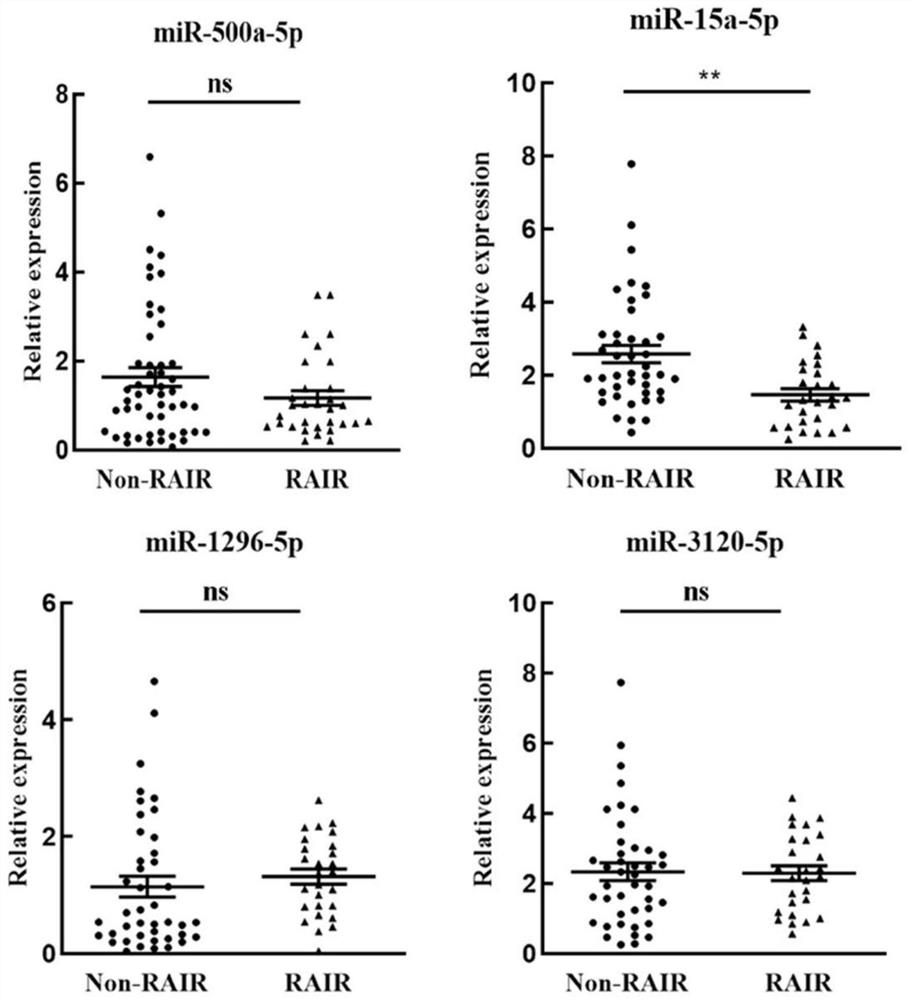
[0057] Experimental Example 1: Clinical Verification
[0058] In this experiment example, the level of exosomal miR-15a-5p in clinical plasma samples was detected, and the levels of miR-500a-5p, miR-1296-5p and miR-3120-5p were used as controls. Among them, miR-500a-5p, miR-1296-5p and miR-3120-5p are miRNAs previously reported to be associated with sodium iodide transporter (SLC5A5, NIS) and tumors.
[0059] 1. Method
[0060] Randomly select 21 plasma samples of iodine-tolerant PTC patients and 42 plasma samples of non-iodine-resistant PTC patients, use the exosome isolation kit ExoQuick-TCTM to extract exosome RNA, use the kit in Example 1 and Methods The level of miR-15a-5p in plasma exosomes was detected, and the primers in Example 1 were replaced with primers of miR-500a-5p, miR-1296-5p and miR-3120-5p to detect miR in plasma exosomes - Levels of 500a-5p, miR-1296-5p and miR-3120-5p. Then the external reference Cel-miR-39 was used to quantify the aforementioned miRNAs. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com