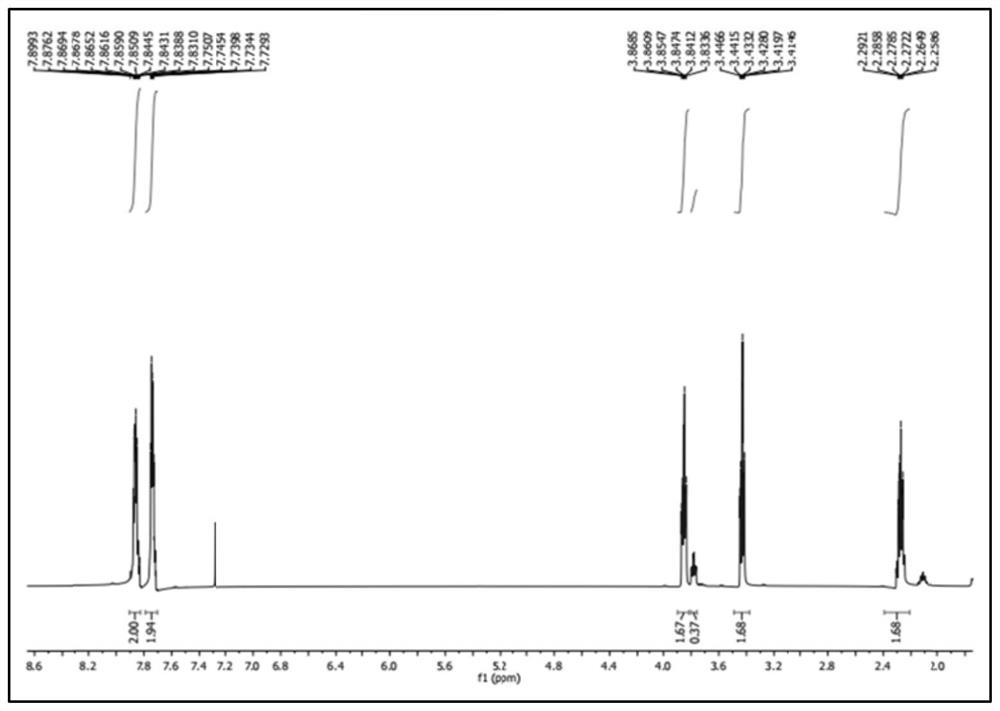
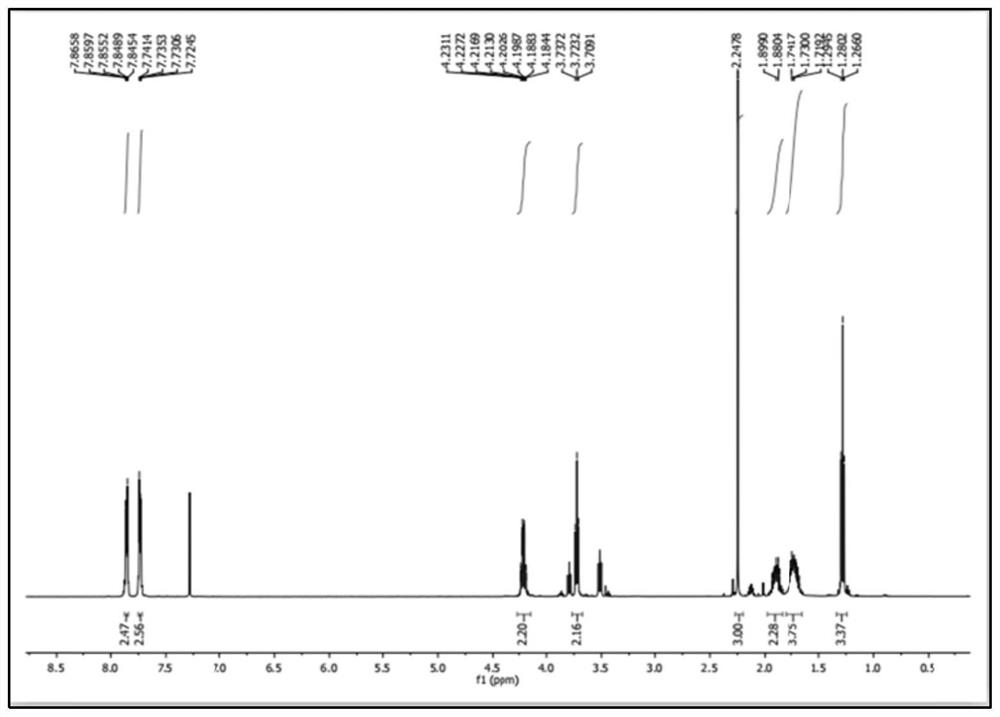
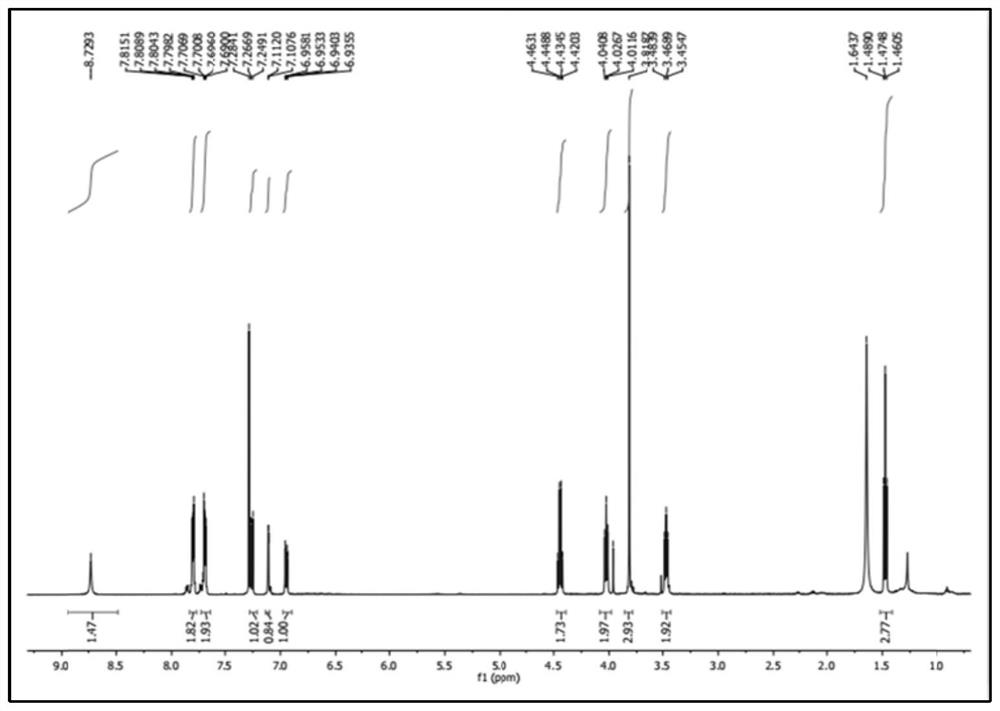
Chemical synthesis method of melatonin
A chemical synthesis, melatonin technology, applied in the field of chemical synthesis of melatonin, can solve the problems that are not suitable for industrial production, many by-products, complicated operations, etc., and achieve mild conditions, few side reactions, and safe reaction reagents Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075]
[0076] 1) Add 350g of 1,3-dibromopropane, 500mL of acetone, and 180g of potassium carbonate to a 3L three-necked flask equipped with a mechanical stirring device, and heat it to 55°C in a water bath to reflux. Add 60 g of phthalimide in batches on average every half hour. Cool to room temperature, filter to remove inorganic salts. Concentrate under reduced pressure to recover acetone. Use an oil pump to distill under reduced pressure to recover 1,3-dibromopropane. The obtained solid was dissolved in 300 mL of absolute ethanol at 70°C, and slowly cooled and crystallized with stirring until -5°C for 1 h. The crystals were filtered, washed with cold ethanol, and dried under vacuum to obtain white crystals of N-(3-bromopropyl)phthalimide with a yield of 98.2% and a purity of 95%.
[0077]
[0078] 2) Add 120 mL of sodium ethoxide solution, 100 mL of ethanol, and 35 mL of ethyl acetoacetate into a three-necked flask equipped with a mechanical stirring device, and ...
Embodiment 2
[0087] 1) Add 400g of 1,3-dibromopropane, 600mL of acetone, and 200g of potassium carbonate to a 3L three-neck flask equipped with a mechanical stirring device, and heat it to 56°C in a water bath to reflux. Add 70 g of phthalimide in batches on average every half hour. Cool to room temperature, filter to remove inorganic salts. Concentrate under reduced pressure to recover acetone. Use an oil pump to distill under reduced pressure to recover 1,3-dibromopropane. The obtained solid was dissolved in 500 mL of methanol at 70°C, and slowly cooled and crystallized while stirring, until kept at -3°C for 1 h. The crystals were filtered, washed with methanol, and dried under vacuum to obtain white crystals of N-(3-bromopropyl)phthalimide with a yield of 98.6% and a purity of 96%.
[0088] 2) Add 150 mL of sodium ethoxide solution, 120 mL of ether, and 50 mL of ethyl acetoacetate into a three-necked flask equipped with a mechanical stirring device, and stir at room temperature. 85 ...
Embodiment 3
[0094] 1) Add 300g of 1,3-dibromopropane, 400mL of acetone, and 150g of potassium carbonate to a 3L three-necked flask equipped with a mechanical stirring device, and heat it in a water bath to reflux at 55°C. Add 50 g of phthalimide in batches on average every half hour. Cool to room temperature, filter to remove inorganic salts. Concentrate under reduced pressure to recover acetone. Use an oil pump to distill under reduced pressure to recover 1,3-dibromopropane. The obtained solid was dissolved in 200 mL of anhydrous diethyl ether at 70°C, cooled and crystallized slowly under stirring, until kept at -5°C for 1 h. The crystals were filtered, washed with ether, and dried under vacuum to obtain white crystals of N-(3-bromopropyl)phthalimide with a yield of 98.3% and a purity of 95%.
[0095]2) Add 100 mL of sodium ethoxide solution, 80 mL of methanol, and 30 mL of ethyl acetoacetate into a three-necked flask equipped with a mechanical stirring device, and stir at room temper...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com