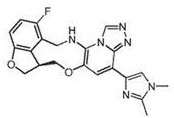
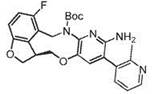
Macrocyclic azolopyridine derivatives as EED and PRC2 modulators
A solvate, C2-C6 technology, applied in the direction of drug combination, medical preparations containing active ingredients, extracellular fluid diseases, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0839] Compound preparation
[0840] The compounds of the present invention can be prepared in a variety of ways well known to those skilled in the art of organic synthesis. By way of example, the compounds of the invention can be synthesized using the methods described below, as well as synthetic methods known in the art of synthetic organic chemistry, or variations thereof understood by those skilled in the art. General methods include, but are not limited to, those described below. In addition, suitable starting materials that are readily available and known to those skilled in the art can be selected to obtain a particular compound of the present disclosure. Compounds of formula I of the present invention can be synthesized according to the steps outlined in Schemes 1-4, wherein R 1 , R 2 and R 3 defined in Formula I. Starting materials are either commercially available or prepared by known procedures in the reported literature or as exemplified.
[0841] plan 1
[...
Embodiment 1
[0937] Example 1: 5-Bromo-6-chloropyridin-3-ol
[0938]
[0939] NaNO at 0°C 2 (31.9 g, 4620 mmol, 1.20 equiv) in water (80.0 mL) was added ice-cold 5-bromo-6-chloropyridin-3-amine (80.0 g, 386 mmol, 1.00 equiv) in H 2 SO 4 (567 g, 2.89 mol, 308 mL, 50% purity, 7.50 equiv), then the mixture was stirred at 25 °C for 30 min. The mixture was added to AcOH (400 mL) at 100 °C. The mixture was stirred at 100 °C for 12 h. LCMS indicated that the reaction was complete. The mixture was concentrated under reduced pressure. The mixture was added to ice water (2000 mL) with saturated Na 2 CO 3 The aqueous solution adjusted the pH to 6-7. The mixture was extracted with EtOAc (5000 mL). The organic layer was washed with brine (2000 mL), washed with Na 2 CO 3 Dry, filter and concentrate under reduced pressure. The residue was subjected to column chromatography (SiO 2 , petroleum ether / ethyl acetate = 1 / 0-4 / 1, petroleum ether / ethyl acetate = 2 / 1, R f = 0.56) purification. ...
Embodiment 2
[0941] Example 2: 5-Bromo-6-chloro-2-nitropyridin-3-ol
[0942]
[0943] Reactions were set up in two separate batches. 5-Bromo-6-chloropyridin-3-ol (46.0 g, 220 mmol, 1.00 equiv) was dissolved in H 2 SO 4 The mixture in (138 mL, 98% purity) was stirred at 0 °C for 75 min. H at 0°C 2 SO 4 (42.3 g, 423 mmol, 23.0 mL, 98% pure, 1.92 equiv) and fuming HNO 3 (19.3 g, 294 mmol, 13.8 mL, 96% purity, 1.33 equiv) was added to the reaction mixture. The mixture was stirred at 0 °C for 2 h. After stirring for 2 h, the mixture was stirred at 20 °C for 12 h. LCMS indicated a small amount of 5-bromo-6-chloropyridin-3-ol remaining and the expected mass detected. The two batches of reaction mixtures were combined and neutralized by adding ice water (3000 mL) and stirred at 20°C for 1 hour. The mixture was filtered and the filter cake was dried under reduced pressure to give 5-bromo-6-chloro-2-nitropyridin-3-ol (83.0 g, crude) as a yellow solid.
[0944]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com