Urine V-ATPASE subunit S1 and application of polypeptide fragment of urine V-ATPASE subunit S1 in allergic diseases
A technology for allergic diseases and polypeptide fragments, which is used in disease diagnosis, material analysis by electromagnetic means, measurement devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 Urine Specimen Collection and Processing
[0021] Patients with allergic diseases were selected as the allergic disease group, and healthy subjects who underwent physical examination during the same period were selected as the normal control group. Collect 30ml of fresh morning urine samples from the research subjects in each group after admission, and collect the urine in the catheter in the morning for those who cannot urinate normally, and place them in a dry and clean container. After the collected urine samples were centrifuged at 4000r / min for 5min, the supernatant was aspirated, divided into 2ml tubes, and stored in a -80°C refrigerator.
Embodiment 2
[0022] Example 2 Mass Spectrometry and Screening of Urine Peptides
[0023] Extract protein from urine samples and determine the concentration of the extracted protein. Mass spectrometric analysis of urine samples was performed by an OrbitrapFusion type mass spectrometer. Quantitative calculation was performed on the data obtained in the mass spectrometer of the experimental group and the normal control group. The difference between groups was analyzed by t-test, and the difference in protein expression was more than 1.5 times and the statistical test P<0.05 was used as the reference standard to screen for differentially expressed proteins.
Embodiment 3
[0024] Example 3 Identification and Analysis of Differential Peptides
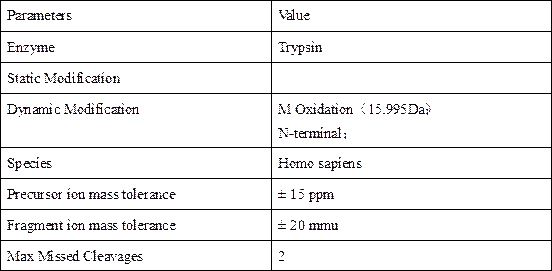
[0025] The database used was the Uniprot_Homo database, and the original mass spectrum files were processed by MaxQuant software. The search parameter settings are shown in Table 1.
[0026] Table 1 Max Quant software retrieval parameter list
[0027]
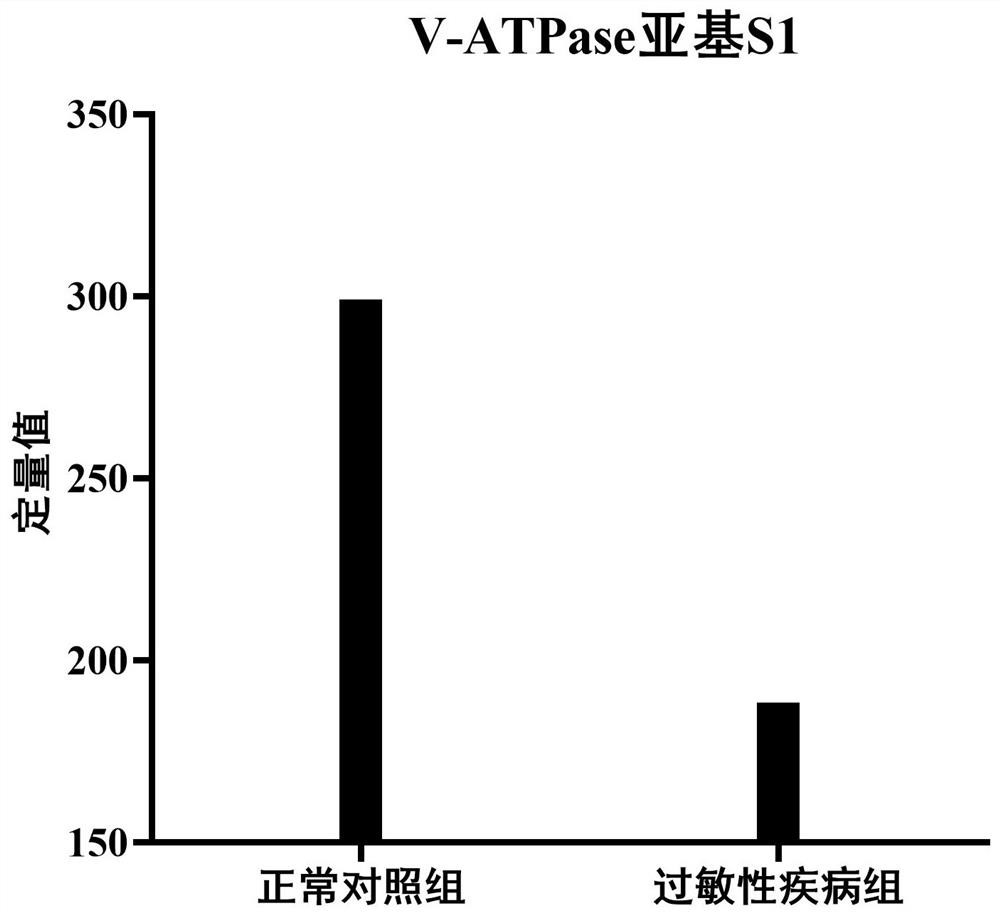
[0028] Compared with healthy people, V-ATPASE subunit S1 was less expressed in the urine of patients with allergic diseases, and its content in the urine of healthy controls and allergic disease groups was as follows: figure 1 As shown, there is a significant difference in the expression of V-ATPASE subunit S1 in the urine of the normal control group and the allergic disease group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com