Application of GeXP multiplex gene expression genetic analysis system in genotyping of 16 common respiratory viruses
A respiratory and viral technology, applied in the field of biotechnology applications, can solve problems such as limited applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
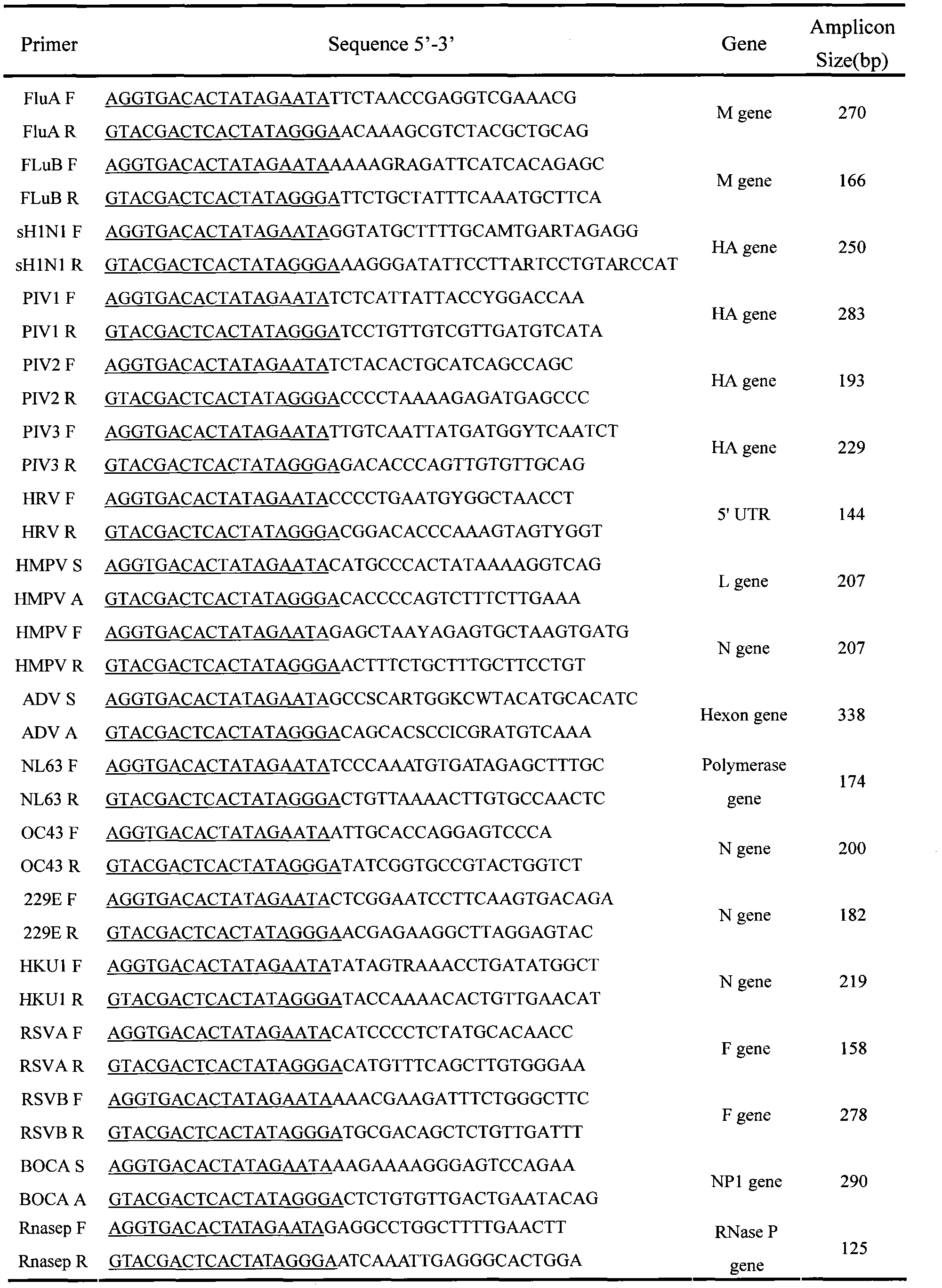
[0015] Embodiment 1: Singleplex RT-PCR Validation Primers
[0016] Single-primer PCR reactions were performed using single-infected positive specimens of multiple strains of known viral nucleic acids as templates, negative clinical samples as negative controls, and redistilled water as blank controls. Qiagen One-step RT-PCR kit was used. 25μl PCR reaction system: 5*buffer 5ul, dNTP Mix 1ul, enzyme mix 1ul, upstream and downstream chimeric primers (1 μmol / L) 1.25 μl each, upstream and downstream universal primers (10 μmol / L) each 1.25 μl, template RNA 2 μl , 0.1ul of RNase inhibitor, make up to 25μl with RNase-free water. The reaction conditions are: reverse transcription at 50°C for 30 minutes; pre-denaturation at 95°C for 15 minutes; specific primer amplification at 95°C for 30s, 55°C for 30s, 72°C for 30s, 10 cycles; chimeric primer amplification at 95°C for 30s, 65°C for 30s, 72°C for 30s, 10 cycles; universal primer amplification at 95°C for 30s, 48°C for 30s, 72°C for 3...
Embodiment approach 2
[0017] Embodiment 2: Multiple reaction system specificity verification
[0018] Prepare multiple mixed primer (Mix-Primer) working solutions, so that the final concentration of each SP-Primer in the RT-PCR system is 50nmol / L, and the rest of the components are the same as those verified by the primers. Using a single positive sample of multiple strains of known viral nucleic acids as a template, a multi-primer PCR reaction is performed.
Embodiment approach 3
[0019] Embodiment 3: Multiple detection system single template sensitivity test
[0020] Use tag-free specific primers to amplify the target nucleic acid region, connect the PCR product to the pGEM-T vector for single cloning, extract the cloned plasmid, digest it with Spe I to linearize it, and use RiboMAX TM Large Scale RNA Production System-T7 reagent The RNA fragments transcribed in vitro were purified by the RNeasy MinElute Cleanup Kit, quantified by a NanoDrop ND-1000 UV spectrophotometer, and the copy number of the RNA was calculated according to the molecular weight and nucleic acid concentration. In vitro transcribed RNA was serially diluted to 10 6 、10 5 、10 4 、10 2 、10 1 copies / μL, take 1 μl each as a template, and test the sensitivity of the multiplex system. Three parallel experiments on different days.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com