Application of sophoridine derivative 6j in preparation of ferroptosis inducer
A technology of sophoridine and its derivatives, applied in the field of application of sophoridine derivative 6j in the preparation of ferroptosis inducers, achieving the effects of increased accumulation, good in vitro anti-hepatoma activity, and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1: This example is a method for producing a preparation of the alkali derivative 6j of the present invention. The structural formula of the 定 碱 碱 6J is as follows:
[0046]
[0047] The specific preparation process is as follows:
[0048] Step 1: Preparation of Compound A
[0049] 10 mmol of raw alkali 2.48 g was refluxed in a 50 ml concentration of 2 mol / L of NaOH aqueous NaOH for 8 h, and the solvent was evaporated to dryness. Under ice bath conditions, 3 ml of SOCL 2 was added to 40 ml of stirring to stir for 30 minutes, and then the crude product was gradually added to the methanol solution of SOCL2, reflux reaction for 3 h. After the reaction, filter with funnel, the organic layer concentrated to compound A.
[0050] Step 2: Preparation of Compound 5A-5P
[0051] Substituted from 5.00 mmol, 1.0eq.r1 inindole in 20 ml N, N-dimethylformamide DMF, under an ice bath, gradually added 216 mg, 5.40 mmol, 1.08Eq NaH, and reacted at room temperature for 30 min, then...
Embodiment 2
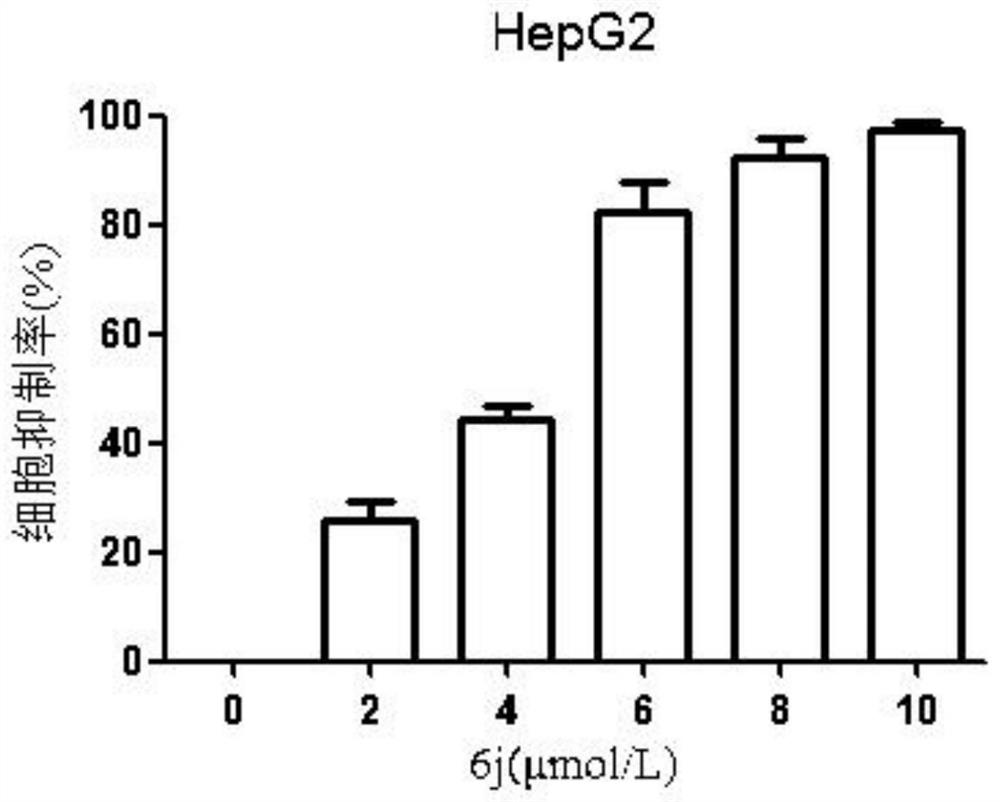
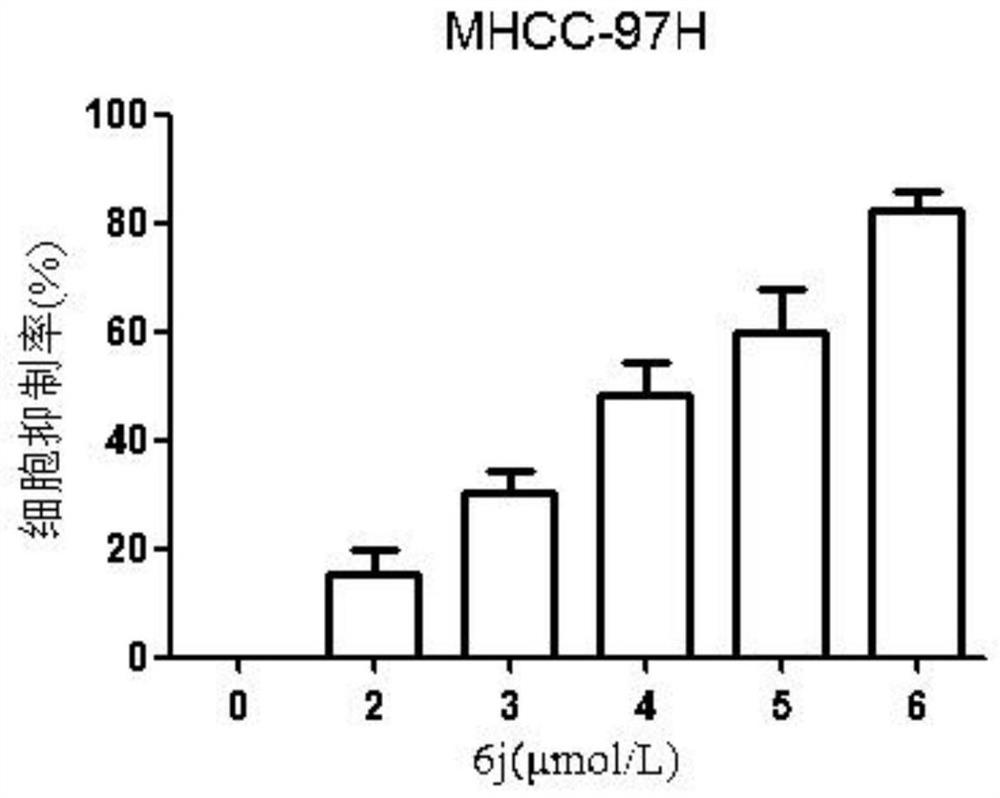
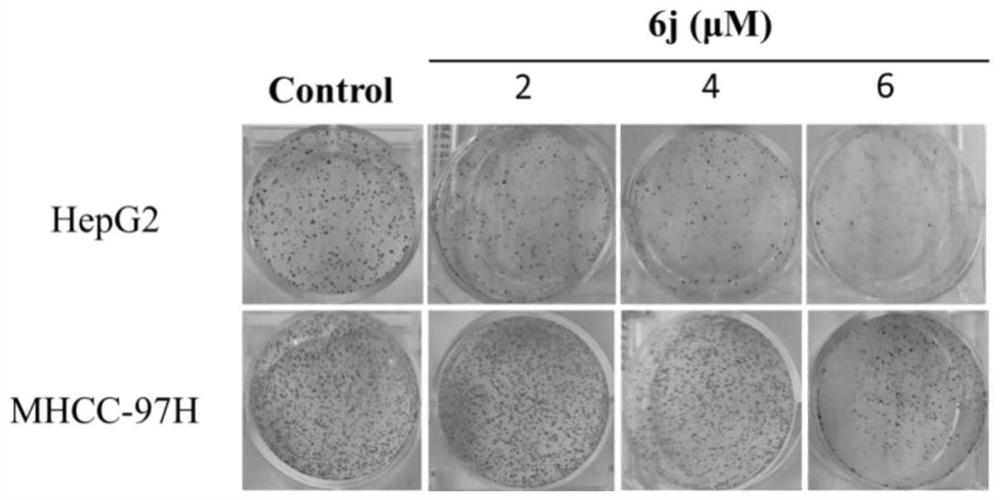
[0056] This example is an embodiment of the proliferative inhibition and migration inhibitory effect of the lactic alkali derivative of the present invention on hepatoma HepG2, MHCC-97H cells, and specific processes are as follows:
[0057] (1) Cell culture
[0058] MHCC-97H and HEPG2 cells are placed at 37 ° C, 5% CO with DMEM medium containing 10% fetal bovine serum, 1% penicillin streptomycin 2 Culture in cell incubator.
[0059] (2) MTT experiment
[0060] The total growth cells were in 5,000 or 100 μl of cells per well. After the cells were stabilized in the culture plate, the fresh-containing medium was changed, and the concentration of the alkali derivative 6j was set to: MHCC-97H (0, 2, 3, 4, 5, 6 μm), HepG2 (0, 2, 4, 6, 8, 10 μm), five complexes per group. After 48 h, each group was treated with 15 μl of MTT (5 g / l), incubated at 37 ° C for 4 h, then the culture solution and MTT mixture were suction, add dimethyl sulfoxide (DMSO) 150 μl / well, in 37 The ° C oven was in...
Embodiment 3
[0068] This example is an embodiment of the inhibitory effect of Iron Death Inhibitor Fer-1 (MCE Company) partially reverse transformer alkali derivative 6j on the inhibition of hepatoma cells HepG2, MHCC-97H, and the specific implementation process is as follows:
[0069] (1) Experimental method
[0070] The total growth cells were in 5,000 or 100 μl of cells per well. After the cell growth is stable, a blank control group, Fer-1 group (1 μm), ragtocytic derivative 6j group (2.5 μm, 5 μm, 7.5 μm), ragtal alkali derivative 6J + Fer-1 group (2.5 μm) + 1 μm, 5 μm + 1 μm, 7.5 μm + 1 μm, Fer-1 is 1 μm), and five additional holes per group. Fer-1 pretreatment cells were added after 6 hours of Litrika derivative 6j + Fer-1 group, and the other group of drugs were treated for 16 h after 16 h after 6 hours. The OD value was detected by the MTT method of Example 2, the suppression rate was calculated, and the experiment was repeated three times.
[0071] (2) Experimental results
[0072] E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com