Composition for whitening skin comprising dihydro-5-methylfuran-2 (3h)-one derivatives
A skin-whitening and cosmetic composition technology, which is applied in the directions of drug combinations, skin-care preparations, active ingredients of hydroxy compounds, etc., can solve problems such as inhibition of melanin production by undisclosed marliolide, and achieve prevention or treatment of hypermelanosis diseases, reduction of Melanin, low risk effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0095] cell culture
[0096] B16 melanoma cells were diluted with 0.25% trypsin solution and separated. The separated cells were added to Dulbecco's modified eagle's medium (Dulbecco's modified eagle's medium) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin / streptomycin (100 U / ml). , DMEM), at 37°C, 5% CO 2 Adapted and cultured in an incubator.
Embodiment 1
[0098] Analysis of Effects on Cell Survival
[0099] The method of measuring cytotoxicity by MTT assay (MTT assay) using B16 melanoma cells is as follows.
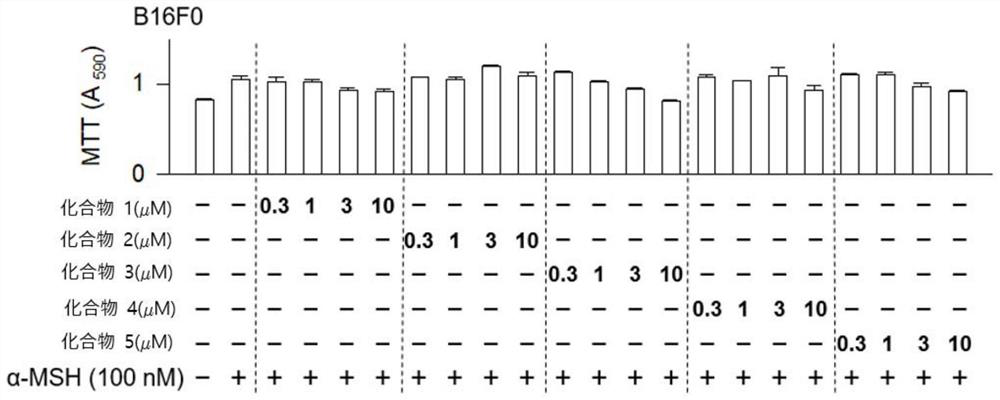
[0100] 200 μL of B16 melanoma cells in 2.7 × 10 3 Cells / well (cells / well) were seeded in a 96-well plate (96well plate), and then incubated at 37°C, 5% CO 2 incubator for 24 hours. Compounds 1 to 5 were treated with B16 melanoma cells at concentrations of 0.3 μM, 1 μM, 3 μM, and 10 μM for 2 hours.
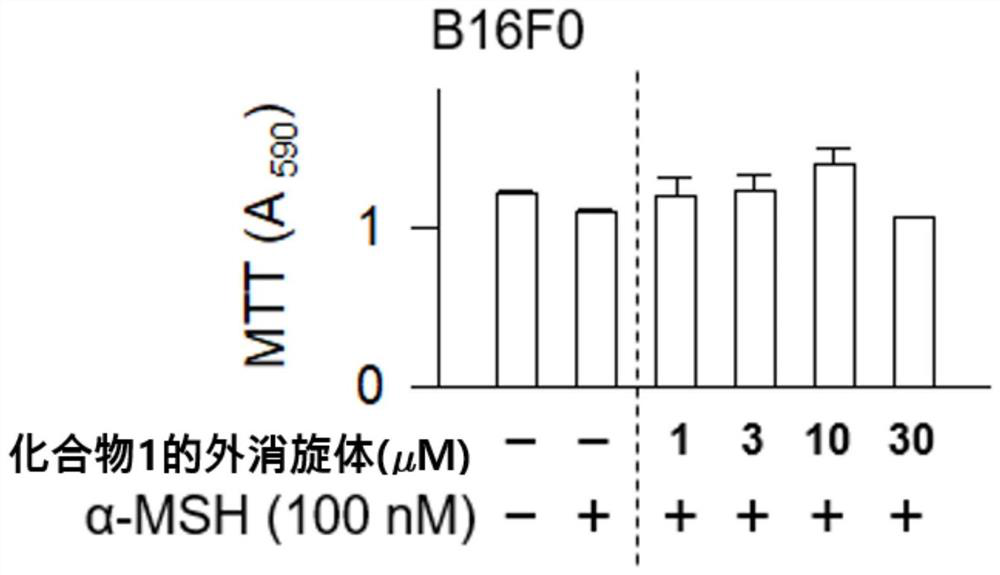
[0101] Then, α-MSH was treated with a concentration of 100nM for 76 hours, and the absorbance at 590nm was measured by the MTT method, and the results were as follows: figure 1 shown. And, for the racemic mixture of Compound 1 at 1 μM, 3 μM, and 10 μM, the absorbance was measured by the same method, and the results were as follows: figure 2 shown.
[0102] Such as figure 1 and figure 2 As shown, compared with the control group, the compounds of the present invention all exhibited the same level of cell viability, thus...
Embodiment 2
[0104] Melanin production inhibition and melanosome decomposition effect
[0105] Using B16 melanoma cells, the effects of melanin production inhibition and melanosome decomposition were confirmed by the following method.
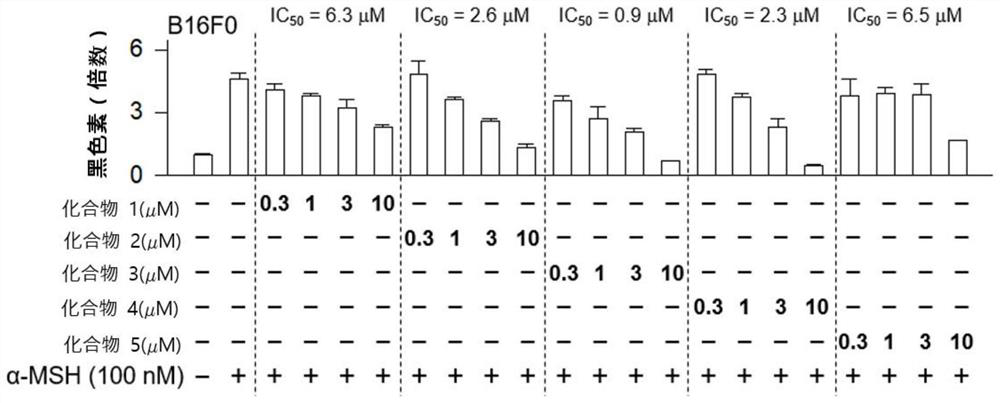
[0106] B16 melanoma cells in 2.7×10 3cells / well were seeded in a 96-well culture plate. After 24 hours, the culture medium of the 96-well was removed, and the DMEM medium containing 10% FBS and compound 1 to compound 5 were treated at concentrations of 0.3 μM, 1 μM, 3 μM, and 10 μM for 2 hours. Then, α-MSH was treated at a concentration of 100 nM for 72 hours.
[0107] After 72 hours, measure the amount of melanin released into the culture medium by measuring the absorbance at 405 nm, the results are as follows image 3 shown. And, for the racemic mixture of Compound 1 at 1 μM, 3 μM, 10 μM, and 30 μM, the absorbance was measured by the same method, and the results were as follows: Figure 4 shown.
[0108] Such as image 3 and Figure 4 As shown, co...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap