Arachidonic acid-hyaluronic acid conjugate as well as preparation method and application thereof
A technology of arachidonic acid and hyaluronic acid, which is applied in the directions of drug combination, pharmaceutical formulation, and inactive medical preparations, etc., to achieve the effects of improving bioavailability and safety, promoting apoptosis, and inhibiting migration and invasion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
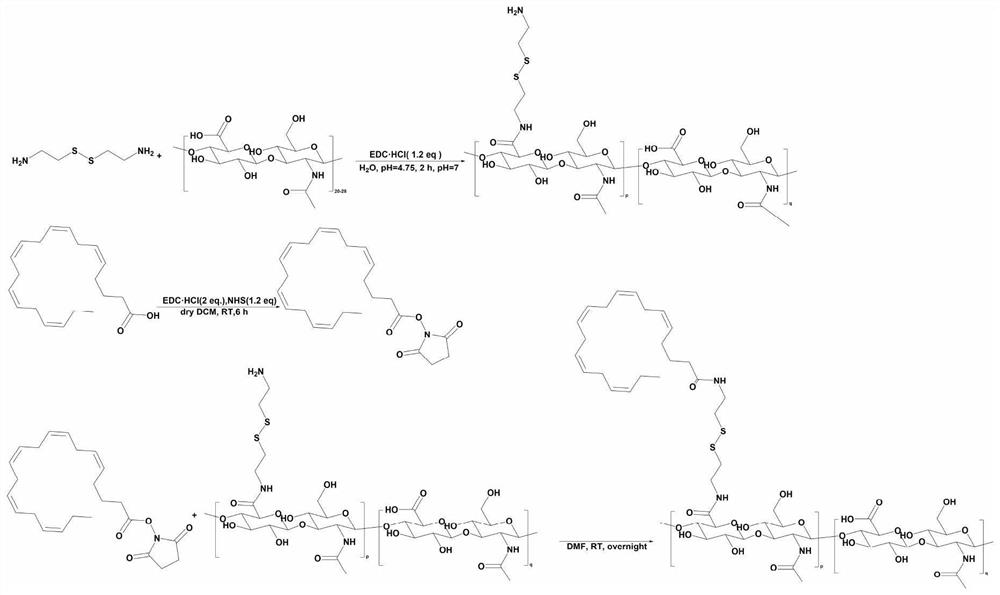
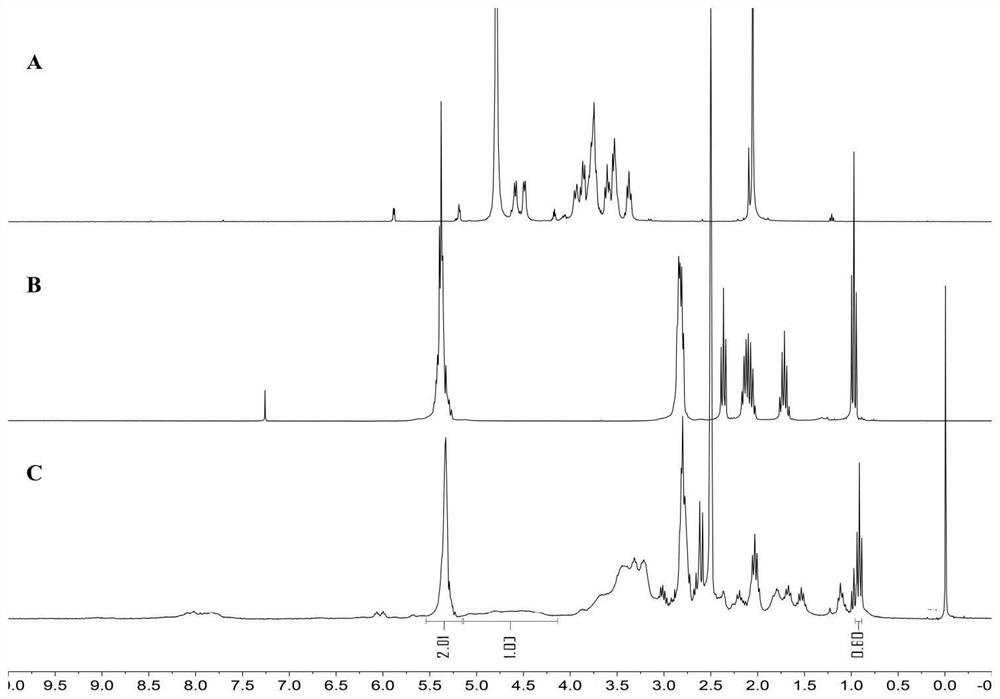
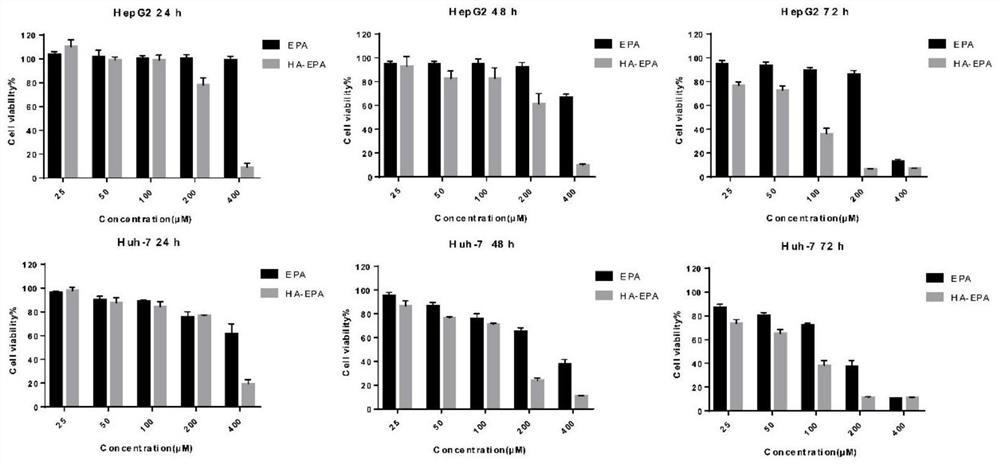
[0039] The preparation method of hyaluronic acid-eicosapentaenoic acid (HA-EPA) of the present embodiment, its synthetic route is as follows figure 1 As shown, the specific steps are as follows:
[0040] Synthesis of Hyaluronic Acid-Amine (HA-cys-amine)
[0041] Accurately weigh 20 mmol sodium hyaluronate and dissolve in deionized water, add 1.2 equivalents of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl), 3 equivalents Cystamine dihydrochloride and 2 M HCl were used to adjust the pH to 4.75. After stirring at room temperature for 2 h, 1 N NaOH was added to adjust the pH to 7 to terminate the reaction. The reaction solution was placed in a 8000 Da dialysis bag, dialyzed against deionized water for 48 h, and the dialysis water was changed every 4 h. After dialysis, the liquid was freeze-dried for 24 hours to obtain a milky white fluffy solid.
[0042] Synthesis of Eicosapentaenoic Acid Succinate Monoester Activator (EPA-NHS)
[0043] Dissolve 0.5 mmol...
Embodiment 2
[0047] The preparation method of hyaluronic acid-eicosapentaenoic acid (HA-EPA) of the present embodiment, its synthetic route is as follows figure 1 As shown, the specific steps are as follows:
[0048] Synthesis of Hyaluronic Acid-Amine (HA-cys-amine)
[0049] Accurately weigh 20 mmol sodium hyaluronate and dissolve it in deionized water, add 5 equivalents of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl), 5 equivalents successively Cystamine dihydrochloride and 2 M HCl were used to adjust the pH to 4.75. After stirring at room temperature for 2 h, 1 N NaOH was added to adjust the pH to 7 to terminate the reaction. The reaction solution was placed in a 8000 Da dialysis bag, dialyzed against deionized water for 48 h, and the dialysis water was changed every 4 h. After dialysis, the liquid was freeze-dried for 24 hours to obtain a milky white fluffy solid.
[0050] Synthesis of Eicosapentaenoic Acid Succinate Monoester Activator (EPA-NHS)
[0051] Dis...
Embodiment 3
[0055] The preparation method of hyaluronic acid-eicosapentaenoic acid (HA-EPA) of the present embodiment, its synthetic route is as follows figure 1 As shown, the specific steps are as follows:
[0056] Synthesis of Hyaluronic Acid-Amine (HA-cys-amine)
[0057] Accurately weigh 20 mmol sodium hyaluronate and dissolve in deionized water, add 0.5 equivalent of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC·HCl), 1 equivalent Cystamine dihydrochloride and 2 M HCl were used to adjust the pH to 4.75. After stirring at room temperature for 2 h, 1 N NaOH was added to adjust the pH to 7 to terminate the reaction. The reaction solution was placed in a 8000 Da dialysis bag, dialyzed against deionized water for 48 h, and the dialysis water was changed every 4 h. After dialysis, the liquid was freeze-dried for 24 hours to obtain a milky white fluffy solid.
[0058] Synthesis of Eicosapentaenoic Acid Succinate Monoester Activator (EPA-NHS)
[0059] Dissolve 0.5 mmol E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com